Introduction
Species in the genus Spodoptera (Lepidoptera: Noctuidae) are major pests of a diversity of crops in different parts of the world (Pogue, 2002). The beet armyworm, Spodoptera exigua (Hübner) and the fall armyworm, Spodoptera frugiperda (J.E. Smith) are the most important and widely distributed species in this genus. The larvae of S. exigua feed on numerous horticultural crops including chili pepper, celery, lettuce, tomato, bean, alfalfa, beet and others (Greenberg et al., 2001). This pest has a world-wide distribution. In contrast, larvae of S. frugiperda primarily attack crops such as maize, rice, sorghum and sugarcane, although larvae are also highly polyphagous and can feed on many other plants (Tay et al., 2023). This pest is native to the Americas but has spread across Africa and Asia over the past decade and is currently invading Oceania (Kenis et al., 2023). The pest is estimated to cause crop losses exceeding 9 billion dollars per year in sub-Saharan Africa alone (Eschen et al., 2021).
The control of both these species is mainly achieved using synthetic insecticides such as organophosphates and pyrethroids, or selective biorational alternatives such as spinetoram, chlorantraniliprole, or insect growth regulators (Paredes-Sánchez et al., 2021; Hafeez et al., 2022). However, both pests have developed important levels of resistance to the most commonly used insecticides (Hilliou et al., 2021). The intensive use of broad-spectrum insecticides can also adversely affect beneficial insect populations (Serrão et al., 2022) and impact farmer health if applied without suitable protection measures (Barrientos-Gutiérrez et al., 2013).
Nucleopolyhedroviruses (genus Alphabaculovirus, family Baculoviridae) are specific pathogens of Lepidoptera that can form the basis for effective biological insecticides (Moore & Jukes, 2019). The virus genome is wrapped inside a protein nucleocapsid and enveloped individually or in groups to form the occlusion derived virion (ODV) (Harrison et al., 2018). Groups of ODVs are occluded within a protein (polyhedrin) matrix to form resistant occlusion bodies that protect the virus and allow it to persist in the environment. Infection begins when a susceptible larva consumes foliage contaminated with OBs. The OBs dissolve in the alkaline midgut, releasing the ODVs that infect midgut cells and then disperse as budded virions to initiate a systemic infection of the insect. Later, nucleocapsids are retained in the cell nucleus to form progeny ODVs and OBs that are released in massive numbers for transmission following the death of the insect (Haase et al., 2015; Williams, 2018). Both Spodoptera frugiperda multiple nucleopolyhedrovirus (SfMNPV) and Spodoptera exigua multiple nucleopolyhedrovirus (SeMNPV) have proven to be effective active ingredients for biological insecticides and are being used in different parts of the world (Lasa et al., 2007a; Haase et al., 2015; Sun, 2015).
One of the limitations to the commercialization of nucleopolyhedrovirus-based insecticides is the need to produce the viruses in living insects (Lacey et al., 2015). Although most of these viruses are amenable to replication in cell culture, they rapidly adapt to in vitro conditions and tend to lose virulence towards their insect hosts (Reid et al., 2014). As a result, all the currently available nucleopolyhedrovirus-based insecticides are produced industrially by inoculation of large numbers of larvae from insect colonies followed by incubation on a semisynthetic diet and harvesting and extraction of the OBs produced in virus-killed insects (Grzywacz & Moore, 2017).
The peritrophic matrix is a complex chitinous tubular structure produced in the lepidopteran midgut to protect the larval intestine from physical abrasion and pathogenic microorganisms (Erlandson et al., 2019). Nucleopolyhedrovirus ODVs released in the midgut have to traverse the peritrophic matrix to gain access to midgut epithelial cells. In a series of studies on the nucleopolyhedrovirus (BmNPV) of the silkworm, Bombyx mori L., exposure of fifth instar larvae to diet containing the chitin synthesis inhibitor flufenoxuron resulted in reduction in the lethal dose of BmNPV OBs of between one and six logarithms compared to control insects (Arakawa, 2002; Arakawa & Sugiyama, 2002). This effect was also observed when larvae were administered as budded virions (Arakawa et al., 2002) and was attributed to the inhibition of chitin synthesis in midgut cells, resulting in a fragile and porous peritrophic matrix in flufenoxuron-treated insects (Arakawa, 2002).
A dramatic reduction in the dose of OBs required to initiate a lethal infection would greatly improve the efficacy of these viruses as biological insecticides and also reduce the costs of production as lower quantities of OBs would be required to provide adequate crop protection. We therefore examined the effect of low-concentration flufenoxuron treatment on the susceptibility of S. exigua and S. frugiperda larvae to their homologous nucleopolyhedroviruses. We also examined the effects of this compound on the production of progeny OBs in each species to determine whether it could be used to increase the productivity of these viruses in insects, as reported previously for other types of insect growth regulators (Lasa et al., 2007c).
Materials and methods
Insects, nucleopolyhedroviruses and insect growth regulator. Larvae of S. exigua and S. frugiperda were obtained from laboratory colonies maintained in the Instituto de Ecología A.C., Xalapa, Veracruz. Both colonies were started with individuals kindly provided by A.M. Martínez-Castillo (Universidad Michoacana de San Nicolás Hidalgo, Michoacán). The larvae were reared individually in 30 ml plastic pots with a semisynthetic diet comprising wheatgerm, soybean flour, brewer's yeast, agar and vitamins adapted from Mihm (1984). Pupae were surface decontaminated in 0.1% sodium hypochlorite solution, washed with tap water, dried on a paper towel and allowed to emerge in 500 mL plastic cups. Groups of (20 adults were placed in paper bags for mating and oviposition. Adults had continuous access to 10% honey solution on a cotton pad.
The isolate of SfMNPV (Sf-NIC) originated from Nicaragua and has been characterized in detail (Escribano et al., 1999; Simón et al., 2011). The isolate of SeMNPV (Se-US2) was kindly gifted by P. Tamez-Guerra (Universidad Autónoma de Nuevo León, Monterrey) and was genetically similar in terms of restriction endonuclease profiles to the active ingredient of the commercial insecticide Spod-X® characterized previously (Muñoz et al., 1999).
A pure preparation of the insect growth regulator (IGR) and chitin synthesis inhibitor flufenoxuron (N-{4-[2-Chloro-4-(trifluoromethyl) phenoxy]-2-fluorophenylaminocarbonyl}-2,6-difluoro-benzamide; 98% purity) was obtained from Sigma-Aldrich (St. Louis, USA).
Insect rearing and all laboratory experiments were performed at 27 ± 1 °C, 70 ±10% relative humidity and a 14 h: 10 h light:dark photoperiod.
Production of stock virus suspensions. To produce the viruses, small pieces of diet were treated with 1 × 109 OB/ml of each virus in 24 well cell culture plates. Fourth instars of each species were allowed to feed on the diet for 5 - 7 days, after which larvae showing signs of polyhedrosis disease were triturated in distilled water. The resulting suspension was filtered through 80 µm steel mesh to remove debris and the OB concentration was determined by counting in triplicate in an Improved Neubauer chamber using a phase contrast microscope at x400 (Muñoz et al., 2001). OB suspensions were stored at 4 °C until required for experiments.
To confirm the identity of each virus, the restriction endonuclease profile of each virus was determined. For this, 3xDAS alkaline buffer (0.5 M NaCl; 0.3 M Na2CO3, 30 mM EDTA; pH 10.5) was mixed with OB suspension in a 1:2 ratio and incubated for 60 min at 45 °C to release ODVs. Debris was pelleted at 3000 rpm for 5 min and the supernatant was centrifuged at 12,000 rpm for 10 min to pellet ODVs. The ODVs were then resuspended in 100 µL sterile distilled water with 10 µL of 10% sodium dodecyl sulfate (SDS) and 3 µL of proteinase K (20 mg/mL), and incubated at 45 °C for 60 min. Viral DNA was extracted by phenol-chloroform treatment and precipitated in ethanol and 3 M sodium acetate at -20 °C. The resulting DNA pellet was washed with 70% ethanol, resuspended in 50 µL MilliQ water and a 1 µg sample from each virus was digested with EcoRI (New England Biolabs, Ipswich, MA) at 37 °C following the manufacturer's recommendations. Samples were then loaded in a 0.7% agarose gel in TBE buffer (100 mM Tris, 90 mM boric acid, 1 mM EDTA; pH 8.3) containing 35 µl/l of ethidium bromide (10 mg/mL) and were subjected to electrophoresis at 30 V overnight. The restriction profile of the amplified OBs of SeMNPV was compared to the original sample supplied by Dr. P. Taméz-Guerra, whereas the amplified OBs of SfMNPV were compared with SfNIC genotype B (SfNIC-B) supplied by Dr. O. Simón (Universidad Pública de Navarra, Spain). SfNIC-B is the majority genotype in the Nicaraguan SfMNPV isolate (Simón et al., 2011). The gel was then photographed on a transilluminator (UV ChemiDoc XRS + Image Lab Software; Bio-Rad, Hercules, CA).
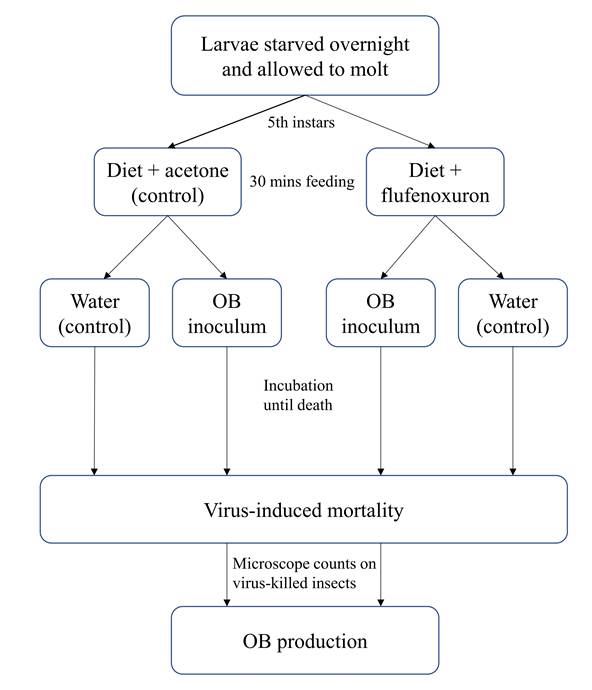
Determination of sublethal concentrations of virus and flufenoxuron. As flufenoxuron is a commercial insecticide, it was first necessary to determine the concentration that would result in a low level of insect mortality. For this, 10 mg flufenoxuron was diluted in 10 ml acetone (equivalent to 1000 mg/l) to produce a stock solution, as this compound does not have high activity in water or as dry powder (Arakawa, 2002). A 5 mm deep layer of semisynthetic diet was prepared in plastic dishes (10 cm diameter, 4 cm height) and was surface contaminated by applying 1 ml of flufenoxuron diluted to concentrations of 100, 10 and 1 mg/l. The solution was spread uniformly over the diet surface and allowed to dry for 30 min. Control dishes were treated with 1 ml of acetone alone. For each species, a group of 15 fifth instars that had been starved for the previous 24 h was placed in each dish and allowed to feed on the diet for 30 - 40 min. The larvae were then placed individually in 30 ml plastic pots with a piece of uncontaminated diet and monitored for mortality at 24 h intervals for 12 days. Each flufenoxuron concentration and controls were replicated across three batches of insects on different days.
To estimate the virus concentrations that would result in low levels (15 - 30%) of lethal infection, diet contamination bioassays were performed. For this, dishes containing a 5 mm thick layer of diet were treated with 1 ml acetone and allowed to dry for 30 min. Fourth instar larvae of each species were starved overnight and allowed to molt to the fifth instar. Groups of 15 larvae in the fifth instar were then placed in dishes and allowed to feed for 30 min (to mimic the exposure to residual traces of acetone described above). Larvae were then transferred individually to 30 ml plastic pots containing a 3 mm layer of diet (surface area 706 mm2) that had been surface contaminated with 50 µl of one of the following three concentrations of OBs: 4.3 × 103, 1.5 × 104, 4.6 × 104 OBs/ml of SeMNPV OBs and 1.3 × 106, 6.5 × 106, 1.3 × 107 OBs/ml of SfMNPV OBs. Control insects were provided with diet that had been treated with 50 µl distilled water. Insects were allowed to feed and develop and were checked at daily intervals to determine the prevalence of lethal polyhedrosis disease for 12 days. Virus-induced deaths were confirmed by observing OBs in Giemsa-stained smears of insect tissues using a phase-contrast microscope. Each OB concentration was replicated across three batches of insects inoculated on different days.
Effect of flufenoxuron on host susceptibility to SeMNPV and SfMNPV OBs. To determine the effect of an initial exposure to flufenoxuron on larval susceptibility to the homologous nucleopolyhedrovirus, larvae were starved overnight and allowed to molt to the fifth instar. Larvae were randomly assigned to one of three treatments: (i) flufenoxuron in acetone followed by OB inoculation, (ii) acetone-treated diet followed by OB inoculation and (iii) acetone-treated diet followed by mock (water) inoculation as a negative control (Fig. 1). For this, groups of 30 larvae were placed in a plastic dish containing a 5 mm layer of diet that had been surface-treated with 1 ml of acetone containing 1 mg flufenoxuron (estimated to result in (15% mortality in the preliminary bioassays), or acetone alone. The acetone was given 40 min to evaporate prior to introducing the larvae. Larvae were allowed to feed for 30 min and were then individually transferred to 30 ml plastic pots containing a 3 mm layer of diet that had been previously surface-contaminated with 50 µl suspension of 1.5 × 104 OBs/ml of SeMNPV OBs or 1.3 × 10⁶ OBs/ml of SfMNPV OBs, or a distilled water control. Each group of larvae was monitored at 24 h intervals until death or pupation. Virus-killed larvae were frozen individually in microcentrifuge tubes and stored at -20 °C. In cases of doubt, death due to polyhedrosis disease was confirmed by microscopic examination of Giemsa-stained smears of larval tissues. The experiment was performed on six occasions with different batches of insects to give six replicates of each treatment.
Effect of flufenoxuron on OB production. To determine the possible effect of flufenoxuron treatment on OB production, 30 virus-killed larvae were selected at random from those that died in the previous experiment. Each larva was placed in a microcentrifuge tube with 800 µl of distilled water and triturated using a pipette tip. The resulting suspension was filtered through a 50 µm pore nylon mesh. The debris collected on the filter was washed with 200 µl distilled water to recover additional OBs. The OB suspension and washing suspension were mixed, using a vortex and OBs were counted in triplicate in a Neubauer Improved chamber (Brand, Wertheim, Germany) using a phase contrast microscope at x400.
Statistical analyses. Due to differences in homoscedasticity, the mortality of larvae treated with flufenoxuron or nucleopolyhedrovirus OBs in preliminary bioassays was analyzed by Kruskal-Wallis test and analysis of variance (ANOVA), respectively. Following ANOVA, means were compared by Tukey test. The percentages of mortality of each species of larvae that had been previously exposed to flufenoxuron or acetone alone were not normally-distributed and were compared by Kruskal-Wallis test and are presented as median values and interquartile range (IQR). The production of OBs per larva was compared by t-test on log-transformed OB counts. In all cases the normality of data was confirmed by Shapiro-Wilk test and homoscedasticity was determined by Levene's test. All analyses were performed using the R-based package Jamovi 2.3.25 (Jamovi, 2023).
Results
Confirmation of virus identity. To ensure fidelity of virus replication viral genomic DNA was digested with the restriction endonucleases EcoRI and electrophoresed in 0.7% agarose (Fig. 2). The experimental material of each of the viruses produced restriction fragment profiles that were identical or near identical to each of the reference virus profiles, indicating that the virus stocks were not degraded and were free from obvious contamination.
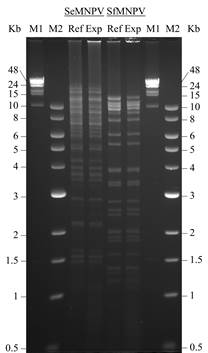
Figure 2 Restriction endonuclease analysis of Spodoptera exigua multiple nucleopolyhedrovirus (SeMNPV) and Spodoptera frugiperda multiple nucleopolyhedrovirus (SfMNPV) following treatment with EcoRI. In both cases, the restriction profile of experimentally amplified OBs (Exp) from the present study was validated against that of a reference isolate (Ref) of each virus. The fragment size markers were lambda-phage DNA-HindIII (M1) and NEB 1 Kb ladder (M2).
Determination of sublethal concentrations of viruses and flufenoxuron. The mortality of S. exigua fifth instars that fed on flufenoxuron contaminated diet increased significantly from 13.3% (interquartile range [IQR] 3.4) at 1 mg/l to 100% (IQR 0) at 100 mg/l (Kruskal-Wallis H = 6.826, d.f. = 2, P = 0.033) (Fig. 3A). Similarly, in S. frugiperda, mortality ranged from 6.6% (IQR 3.3) at 1 mg/l to 100% (IQR 0) in the 100 mg/l treatment (Kruskal-Wallis H = 7.513, d.f. = 2, P = 0.023) (Fig. 3B). No mortality was observed in control insects. The concentration of 1 mg/l of flufenoxuron was therefore selected for subsequent bioassays involving flufenoxuron treatment and virus inoculation.
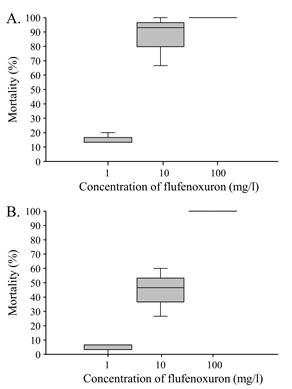
Figure 3 Box-plot showing median mortality of fifth instar larvae of A) S. exigua and B) S. frugiperda following treatment with flufenoxuron.
The results of the bioassays involving nucleopolyhedroviruses revealed a significant increase in virus-induced mortality with increasing inoculum concentration in S. exigua larvae (F = 8.54, d.f. = 2, 6, P = 0.018). Mortality in S. exigua ranged from 24.4% to a maximum of 73.3% across the SeMNPV OB concentrations tested (Fig. 4A). In contrast, virus induced mortality in S. frugiperda ranged from 51% to 62% with increasing SfMNPV OB concentration but this variation was not significant (F = 0.157, d.f. = 2, 6, P = 0.858) (Fig. 4B).
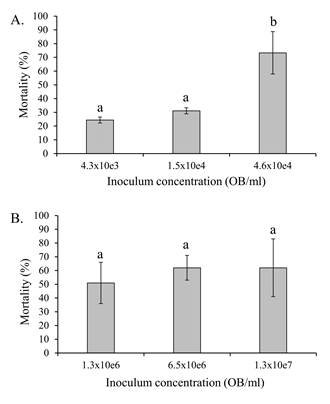
Figure 4 Mean (± SE) percentage of virus-induced mortality of fifth instars of A) S. exigua and B) S. frugiperda following inoculation with different concentrations of SeMNPV or SfMNPV OBs, respectively. Columns headed by identical letters in (A) did not differ significantly (ANOVA, Tukey P > 0.05).
Based on these results, a 1 mg/l concentration of flufenoxuron was selected for the following experiment in combination with OB inoculum concentrations of 1.5 × 104 OBs/ml of SeMNPV OBs or 1.3 × 10⁶ OBs/ml of SfMNPV OBs.
Effect of flufenoxuron on host susceptibility to SeMNPV and SfMNPV OBs. In S. exigua, mean virus-induced mortality ranged between 16.7% and 17.2% but did not differ significantly between larvae that consumed SeMNPV OBs alone or those that were previously treated with flufenoxuron (Paired t = 0.164, d.f. = 5, P = 0.876) (Fig. 5). The mortality of control larvae was 3.3%, but examination of Giemsa-stained larval smears confirmed that this was not due to polyhedrosis disease.
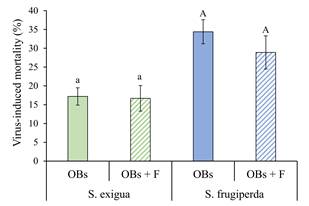
Figure 5 Mean (± SE) percentage of virus-induced mortality of S. exigua and S. frugiperda fifth instars inoculated with their respective homologous virus OBs alone (OBs, solid columns), or following treatment with flufenoxuron (OBs + F, hatched columns). Columns headed by identical letters (S. exigua/SeMNPV, lowercase; S. frugiperda/SfMNPV, uppercase) did not differ significantly (paired t-test, P > 0.05).
Similarly, for S. frugiperda, virus induced mortality varied between 28.9% and 34.4%, but did not vary for larvae that consumed SfMNPV OBs alone or following flufenoxuron treatment (Paired t = 0.977, d.f. = 5, P = 0.374) (Fig. 5). Mortality in the control group did not exceed 3.0%, but this was not due to virus infection.
Effect of flufenoxuron on OB production. The mean production of OBs in S. exigua varied from 1.23 × 109 to 1.62 × 109 per larva, compared to 1.02 × 109 - 1.18 × 109 OBs/larva in S. frugiperda (Fig. 6). Prior treatment with flufenoxuron did not significantly affect the mean production of OBs for either SeMNPV in S. exigua larvae (t = 0.878, d.f. = 10, P = 0.400) or SfMNPV in S. frugiperda larvae (t = 0.774, d.f. = 10, P = 0.457).
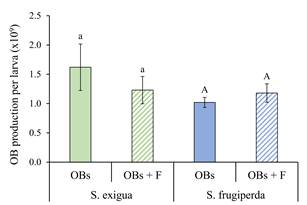
Figure 6 Mean (± SE) occlusion body (OB) production in larvae of S. exigua and S. frugiperda inoculated with their homologous OBs alone (OBs; solid columns), or OBs following larval treatment with flufenoxuron (OBs + F; hatched columns). Columns headed by identical letters (S. exigua/SeMNPV, lowercase; S. frugiperda/SfMNPV, uppercase) did not differ significantly (t-test, P > 0.05).
Discussion
The present study aimed to determine whether sublethal treatment with the chitin synthesis inhibitor flufenoxuron would affect the susceptibility of larvae to nucleopolyhedrovirus infection or the production of progeny OBs in flufenoxuron-treated insects. We addressed these issues using two species of polyphagous lepidopteran pests of worldwide importance, namely S. exigua and S. frugiperda.
Preliminary studies were performed to establish a suitable concentration of flufenoxuron that would not result in high mortality of treated insects. For both species of Spodoptera, treatment with 1 mg/l of flufenoxuron resulted in median mortality of approximately 7 - 13% and this concentration was selected for subsequent experiments. In contrast, concentrations of 10 or 100 mg/l flufenoxuron resulted in 47 - 100% mortality in both species, with S. exigua notably more sensitive to this compound than S. frugiperda, especially at the 10 mg/l concentration (Fig. 3A,B). These findings contrast markedly with the original report of Arakawa (2002) who exposed B. mori fifth instars to concentrations of between 10 and 500 mg/l of flufenoxuron but did not report any resulting mortality, for reasons that we do not understand.
The experiments with each of the viruses were performed at different inoculum concentrations as these species differ in susceptibility to their respective nucleopolyhedroviruses (Fig. 4A,B). When insects were treated with 1 mg/l flufenoxuron followed by a period of continuous feeding on diet that had been surface-contaminated with their homologous virus, the virus-induced mortality was not affected by the flufenoxuron treatment in either species of insect (Fig. 5). Previously, Arakawa (2002) had reported that the peritrophic membrane of B. mori larvae became extremely fragile after larvae had fed on 100 mg/l of flufenoxuron for 21 hours. This effect was not observed in B. mori larvae that consumed other chitin synthesis inhibitors such as teflubenzuron, lufenuron, chlorfluazuron or diflubenzuron. The peritrophic matrix is a major barrier to virus infection in the insect midgut as ODVs have to penetrate this structure presumably through small fissures or pores in the matrix in order to access and infect midgut epithelial cells (Erlandson et al., 2019). The OBs of some nucleopolyhedroviruses and betabaculoviruses release metalloprotease enzymes in the midgut that increase the porosity of the peritrophic matrix resulting in increased infection of midgut cells (Del Rincón-Castro & Ibarra, 2005; Ishimwe et al., 2015; Yang et al., 2017; Ricarte-Bermejo et al., 2021). However, in the case of the study by Arakawa (2002) it is unclear how B. mori larvae treated with such a high concentration of flufenoxuron managed to survive long enough to become infected and die from BmNPV disease. This represents a conundrum in the case of our study as an increased concentration of flufenoxuron may have had a more pronounced effect on peritrophic matrix integrity in S. exigua and S. frugiperda, but would have resulted in a marked increase in IGR-related larval mortality, as evidenced in the preliminary bioassays (Fig. 3A,B).
Other substances that can effectively degrade the peritrophic membrane include stilbene optical brighteners that inhibit chitin synthesis and also reduce midgut cell sloughing (Washburn et al., 1998; Wang & Granados, 2000). Simultaneous consumption of viral OBs and optical brightener can result in dramatic increases in infection rates, especially in late instars that are usually more resistant to infection (Shapiro & Robertson, 1992; Martínez et al., 2003). These compounds have only been used on an experimental basis (Farrar & Shapiro, 2005; Lasa et al., 2007b; Toprak et al., 2007), and not currently included in commercial formulations of baculovirus-based insecticides.
As with the study on insect susceptibility to infection, prior flufenoxuron treatment had no significant effect on OB production per larva (Fig. 6). This suggests that 1 mg/l flufenoxuron treatment either had no marked physiological effects on virus replication or insect growth, or alternatively the effect on the peritrophic matrix was only slight or transitory. Previous studies on the stilbene calcofluor revealed that the peritrophic matrix of Trichoplusia ni (Hübner) larvae could fully recover its structure two hours after the larvae had ingested a stilbene solution (Wang & Granados, 2000). The findings on flufenoxuron contrast with previous studies involving sublethal concentrations of juvenile hormone mimics, such as pyriproxyfen and methoprene, which resulted in enhanced growth of larvae and a significant increase in OB production in each infected larva (Nordin, 1981; Kolodny-Hirsch et al., 1995; Lasa et al., 2007c). This difference underlines the divergent mode of action of the different classes of IGR-type insecticides.
We conclude that flufenoxuron at a concentration of 1 mg/l failed to potentiate the insecticidal activity or production of OBs of SeMNPV or SfMNPV in their respective hosts. Future studies could examine the effect of prolonged exposure, or treatment with different concentrations of flufenoxuron as a means of increasing larval susceptibility to nucleopolyhedrovirus infection. In addition, as chitin synthesis inhibitors are chemically diverse, future research should examine whether compounds from groups such as the oxazolines, thiazolidines, tetrazines, or thiadiazines (Merzendorfer, 2013), have the ability to enhance the insecticidal activity of these viruses.











 nueva página del texto (beta)
nueva página del texto (beta)