Introduction
The seasonality and rainfall variability in tropical habitats have effects on the ecology of lizards because produce variation on the food availability (Miranda & Andrade, 2003). Food is an essential component of the environment that affects the reproduction of organisms. Both the quality and quantity of food are factors that can influence these aspects. In lizards, seasonal changes in the environment affect the diet (Gadsden & Palacios-Orona, 2000).
Many species of amphibians and reptiles occur in the tropical dry forest of Mexico, including the lizards Sceloporus horridus horridus Wiegman 1834. This species occurs in Guerrero, Morelos, Oaxaca and southern Puebla in the Balsas River Basin in central México (Smith & Taylor, 1950), in several localities of tropical dry forest, from south-central region Morelos, and altitudinally from 800 to 1,700 m (Castro-Franco & Bustos, 1994; Castro-Franco & Bustos, 2003). Although the reproductive ecology, thermoregulation, and habitat use of this species have been previously described (Valdéz-González & Ramírez-Bautista, 2002; Bustos et al., 2013; Valencia-Limon et al., 2014), its diet is only partially known. An examination of 17 stomachs obtained from lizards captured in Jalisco, Mexico, showed that this species consumed insects of the orders Homoptera, Coleoptera, Hymenoptera, Orthoptera and Isoptera (Medica & Arndt, 1976). In stomachs of lizards S. h. horridus captured in Morelos (Castro-Franco, 2002) remains of plants and insects were found, such as formicids, larvae, scarabeids and carabids. Likewise, insects such as ants, coleopterans, larvae, lepidopterans and termites have been observed as components of the diet of S. horridus from Puebla (Serrano-Cardozo et al., 2008). Other lizards of the genus Sceloporus also have a diet of insects (Sceloporus mucronatus, Méndez de la Cruz et al., 1992; Sceloporus undulatus consobrinus, Gadsden-Esparza & Palacios-Orona, 1995; Sceloporus gadoviae, Feria-Ortiz & Pérez-Malváez, 2001). In this study, the diet composition of S. h. horridus (Fig. 1), variation in composition between wet and dry seasons, and trophic overlap between the sexes were examined. In addition, the diet of the Morelos population was compared with published data for the same subspecies from Jalisco and Puebla (Medica & Arndt, 1976; Serrano-Cardozo et al., 2008).
Materials and methods
Lizard specimens were captured in the locality Jagüey, Tlaltizapan Morelos, México (18°47.9′77″ N, 99°06.6′89″ W) at 1,035 m elevation on the border of the protected area Sierra Montenegro-Las Trincheras. The site has patches of original tropical dry forest among fields of sorghum. The climate is semi-tropical with the majority of rain in summer and a minor percentage of winter rain (5%). The average annual precipitation is 900 mm and annual average temperature is 22 °C. The dry season is from November until May, with minor precipitation (5.0 mm) between December and March, and the wet season is from June to October, with the maximum precipitation (200 mm) in September.
The stomach contents of 67 lizards (36 females, snout-vent length (SVL) 86.32 ± (SD) 7.94 mm, and 31 males, SVL 90.66 ± (SD) 12.79 mm were analyzed. Lizards were collected between 10:00 h and 14:00 h, over the period May 2002 to April 2003. Snout vent-length (mm), weight (g) and gender of the preserved specimens were recorded. Each stomach was weighted using a digital scale (precision 0.01 g), first with food (Pf) and then empty (Pt). The difference of Pf and Pt represents the weight of the stomach contents. The contents sorted into groups were identified taxonomically (Morón & Terrón, 1988; Borror et al., 1992).
Proportional consumption (Pi), frequency of occurrence (FOi), relative abundance (RAi), and relative importance (RIF) of each group of food, trophic diversity (Shannon´s diversity index H′) and the overlap of trophic niche between sexes (Ojk) were calculated. A grid of 10 × 10 mm was used to assess consumption percentage of food (Pi). The stomach contents were spread homogeneously inside the grid; the total number of squares covered by food represented 100% of the diet of that individual (Windell & Bowen, 1978; Mendoza-Estrada et al., 2008). The frequency of occurrence was based on the number of times that the various components of the stomach contents were recorded; the number of items of each food group (ne) was expressed as a percentage of the total number of items in the stomach contents (Ne), calculated by the expression FO = [ne/Ne] × 100 (Lagler, 1977). The relative abundance of each food group (RA) was estimated using the weight (g) of each food group divided by the total weight of the stomach contents for each individual. The relative importance of each food group (RIF) was calculated by the expression
where Pi is the proportion of the total stomach contents of food i, RAi the relative abundance food i, and FOi the frequency of occurrence of food i. Trophic diversity was calculated using Shannon’s diversity index (H′).
where Pi is the percent consumption of food i and ln Pi the natural logarithm of Pi. We evaluated niche overlap by sexes and seasons using the index of MacArthur and Levin (Pianka, 1973). The overlap of trophic nic|hes produces a value of zero for completely separate use of resources, and a value of 1 is complete overlap of resource use.
where Ojk is the overlap between males and females, and between wet and dry seasons, is the percent consumption of resource i, by the males j, is the percent consumption of the resource i, by the females k, and n represents the entire number of food resources.
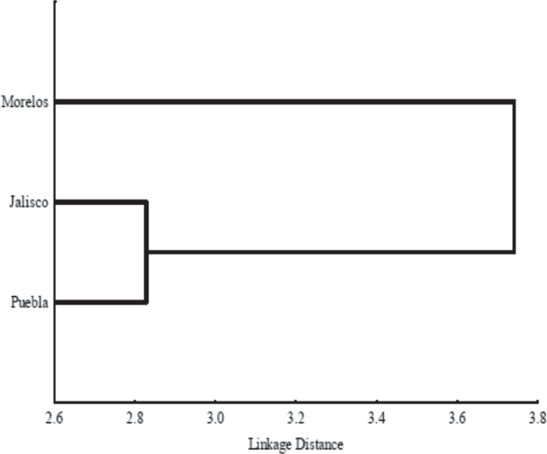
Student’s T-test was used to test for differences in the trophic diversity (H′) of males vs. females, and between wet and dry seasons by using normalized data transformed to log10. A one-way ANOVA with data transformed to log10 was used to test for differences in the weight of the food consumed in wet and dry seasons, and between males and females. Cluster analysis (nearest neighbor distance method) was used with data of presence (1) and absence (0) of food groups, to test the similarity of diets of S. horridus horridus in Morelos (this study), Autlán Jalisco (Medica & Arndt, 1976) and Zapotitlan Valley, Puebla (Serrano-Cardozo et al., 2008). An alpha of P ≤ 0.05 was used in all statistical tests.
Results
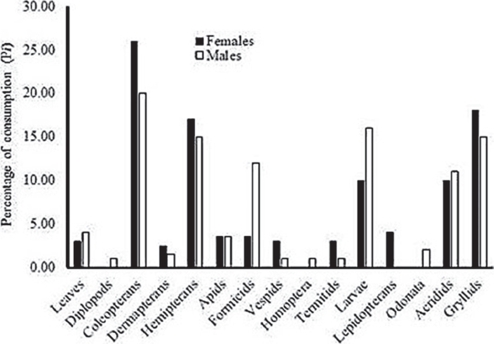
A total of 18 prey items in stomach contents were identified (Table 1) of which 96.0% were invertebrates, and the diversity value was H′ = 4.18. The most frequent groups consumed (FO) were coleopterans (69.3%), hemipterans (68.0%), formicids (38.7%), larvae in general (37.3%) and crickets (25.3%). The remaining food consumed (20.0% of items) corresponded to leaves of Pithecellobium dulce (guamuchil). Coleopterans (21.9%), hemipterans (15.4%), crickets (14.5%), and grasshoppers (12.6 %) were proportionally the major groups consumed by volume (Pi; Table 1).
Table 1 Components of the diets of male (n = 31) and female (n = 36) Sceloporus horridus horridus from Morelos, Mexico. Frequency of occurrence, percentage of consumption, and relative importance of each group of food consumed.
| Prey group | Frequency of occurrence (FO) % | Percentage of consumption (Pi) % | Relative Importance food (RIF) | |||
|---|---|---|---|---|---|---|
| Males (M) | Females (F) | M&F wet season | M&F dry season | |||
| Plant material | ||||||
| (Leaves of Pithecellobium dulce) | 20.0 | 1.9 | 4.0 | 3.0 | 3.6 | 6.3 |
| Diplopods | 2.7 | 0.2 | 0.0 | 2.5 | 1.7 | 0.0 |
| Araneae | ||||||
| Arachnids | 9.3 | 1.4 | 0.0 | 6.7 | 3.6 | 4.5 |
| Scorpionids | 4.0 | 1.1 | 0.0 | 1.2 | 1.7 | 2.1 |
| Insects | ||||||
| Coleopterans | 69.3 | 21.9 | 32.3 | 40.9 | 42.9 | 32.0 |
| Dermapterans | 4.0 | 0.6 | 1.5 | 2.51 | 1.9 | 1.7 |
| Hemipterans | 68.0 | 15.4 | 32.8 | 35.9 | 33.0 | 32.0 |
| Hymenoptera | ||||||
| Apidae | 10.7 | 2.2 | 5.7 | 7.1 | 3.0 | 7.5 |
| Formicids | 38.7 | 6.9 | 36.2 | 16.8 | 18.9 | 31.7 |
| Vespids | 13.3 | 2.9 | 3.6 | 4.3 | 3.8 | 9.3 |
| Homoptera | 2.7 | 0.2 | 2.6 | 0.0 | 0.8 | 1.5 |
| Isoptera (Termites) | 8.0 | 0.6 | 2.5 | 13.2 | 12.3 | 1.7 |
| Larvae in general | 37.3 | 12.8 | 18.5 | 18.0 | 12.8 | 16.9 |
| Lepidopterans | 6.7 | 1.8 | 0.0 | 5.9 | 3.9 | 1.7 |
| Odonata | 1.3 | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 |
| Orthoptera | ||||||
| Grasshopper | 20.0 | 12.6 | 9.3 | 7.8 | 6.6 | 19.5 |
| Crickets | 25.3 | 14.5 | 13.3 | 18.8 | 15.9 | 11.8 |
| Mineral matter (small stones) | 25.3 | 2.2 | 0.0 | 0.0 | 0.0 | 0.0 |
| Indeterminate | 65.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
The food identified in the stomachs of lizards collected in the wet season (n = 46, Fig. 2A), comprised 16 groups, of which coleopterans (80.4%), hemipterans (69.6%), larvae (39.10%), formicids (34.8%) and crickets (28.3%) were the most frequent. In contrast, by volume, the most abundant foods in the stomach in the wet season were coleopterans (28.2%), crickets (15.9%), larvae (14.2%) and hemipterans (13.9%). During the dry season (n = 29), lizards also ate 16 food groups, of which hemipterans (65.51%), coleopterans (58.62%), formicids (44.82%), grasshoppers (34.4 %) and larvae (27.6 %) made up the most frequent food items. The highest proportions of food by volume were grasshoppers (19.91%), hemipterans (18.41%), and crickets (12.08%). Vegetable matter had the lowest proportional consumption (0.69%) in the dry season (Fig. 2B).
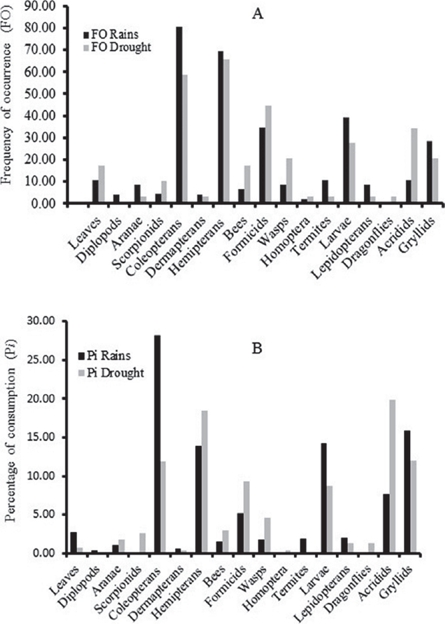
Figure 2 Frequency of occurrence (A), and percent consumption of food (B), in rains and drought in the Mexican endemic lizard, Sceloporus horridus horridus.
The trophic diversity value during the wet season was H′ = 2.09 (T (0,16) = 3.04, P = 0.008) and during the dry season was H′ = 2.23 (T(0,16) = 3.67, P = 0.002). There were significant differences between dry and wet seasons in FO (ANOVA F(1,2) = 183.49, P < 0.0001), and in Pi (ANOVA F(1,2) = 12.72, P = 0.001).
Diet diversity differed significantly between females (H′ = 2.14, T(0,16) = 3.22, P = 0.005, n = 32 stomachs) and males (H′ = 2.08, T(0,16) = 3.39, P = 0.004, n = 32 stomachs). Consumption (Pi) differed significantly between sexes (ANOVA F(1,2) = 21.82, P < 0.0001). Females consumed predominantly coleopterans (26.0%), grasshoppers (17.0%) and hemipterans (17.0%) while males predominantly consumed coleopterans (20.0%), larvae (16.0%), hemipterans (15.0%) and grillids (15.0%). The variation in the diet was related to a high intake of formicids by males (Fig. 3).
Dietary overlap was high between males and females (Ojk = 0.91) and between wet and dry seasons (Ojk = 0.90).
Foods with the highest relative importance were coleopterans (32.3 in males, 40.9 in females) and hemipterans (32.8 in males, 35.9 in females) followed by formicids (36.2 in males, 16.8 in females) and larvae (18.5 in males, 18.0 in females). These foods were important in both wet and dry seasons with some variations in quantity (Table 1).
The cluster analysis showed that the diet composition of lizards from Morelos was different to those of lizards from Jalisco and Puebla (Fig. 4).
Discussion
The diet of the S. horridus horridus from our study area consisted of 18 groups of insects, predominantly coleopterans, hemipterans, formicids, larvae and grasshoppers. This diet composition is different to that observed in the same species of lizards from semi-arid localities with thorny scrub, tropical dry forest and thorny forest (Medica & Arndt, 1976; Serrano-Cardozo et al., 2008). Differences in the quantity of food eaten by lizards are explained by variation of sample size. The contents of 67 stomachs from Morelos lizards were examined, in contrast, data from Jalisco (Medica & Arndt, 1976) and Puebla (Serrano-Cardozo et al., 2008) corresponded to 15 and 16 specimens, respectively. Our results support a diet of opportunist type, previously described for this species (Medica & Arndt, 1976).
In this study, variation in consumption quantities by items was also observed between wet and dry seasons, similarly to other locations and other species of genus Sceloporus (S. horridus, S. gadoviae, S. jalapae) (Medica & Arndt, 1976; Feria-Ortiz & Pérez-Malváez, 2001; Serrano-Cardozo et al., 2008). This variation was a result of increased prey availability in the wet season, and increased consumption of acridids and formicids in the dry season. Such variations have been interpreted as evidence of an opportunistic diet (Medica & Arndt, 1976).
Males and females consumed similar types of food in different proportions, as evidenced by the high values for niche overlap. This is because both adult males and females are arboreal and rupicolous; they are both active between 9:00 h and 16:00 h in the wet season and between 9:00 and 14:00 in the dry season (Bustos et al., 2013). This obviously allows males and females to feed in the same places and at the same periods.
Coleopterans, hemipterans, formicids and larvae were the most important food groups in the S. horridus horridus population from Morelos. In the population of Zapotitlan Valley, Puebla, Mexico (Serrano-Cardozo et al., 2008), insects of the groups Coleoptera, Formicidae, Isoptera (termites) and Lepidoptera (larvae), were also the most important components of the diet. Lizards from Jalisco, fed mainly on homopterans, coleopterans, hymenopteran, orthopterans and isopterans (Medica & Arndt, 1976).
Previous studies (Brooks & Mitchell, 1989; Gadsden & Palacios-Orona, 1997; Feria-Ortiz & Pérez-Malváez, 2001; Pavey et al., 2010; Siqueira et al., 2013) have found that large quantities of termites are also consumed by Sceloporus gadoviae, Cnemidophorus tigris, Uma paraphygas, Uta stansburiana, S. clarki, S. nelson, Egernia slateri and Tropidurus torquatus. This is explained by the abundance of ants and termites in tropical arid and semi-arid areas (Evans et al., 2011; Alí et al., 2013).











 nueva página del texto (beta)
nueva página del texto (beta)