INTRODUCTION
Marijuana is a preparation of the Cannabis sativa herb consumed since ancient times with therapeutic purposes and as a misused drug. Cannabinoids are lipid compounds interacting with cannabinoid receptors. Plant-derived cannabinoids are termed phytocannabinoids and around 100 of them are recognized as exclusive of C. sativa, with Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD) being the most abundant and studied1. The isolation of Δ9-THC allowed its identification as the compound responsible for the psychoactive actions of Cannabis, the characterization of its intoxicating and therapeutic effects, and led to the discovery of the endocannabinoid system (ECS) in vertebrates. The phytocannabinoids Δ9-THC and CBD exert their effects by interacting with the ECS2.
Cannabis preparations meet the criteria to be considered misused drugs, since they induce acute intoxication that can be rewarding and might lead to their repeated intake, producing tolerance to several of its effects and the development of physical dependence, expressed by a withdrawal syndrome. Cannabis use disorder (CUD) is recognized in the diagnostic and statistical manual (DSM-5)3. Cannabis consumption is worldwide spread; it is one of the most consumed drugs with abuse potential, only after alcohol and tobacco. Although considered as an illicit substance for decades, Cannabis legal status is changing to that of a regulated substance in several countries. Otherwise, the therapeutic application of cannabinoid-like compounds for distinct medical conditions is a relevant research field.
To date, the Food and Drug Administration of the United States (FDA) has approved three Cannabis-based pharmaceuticals as therapeutic agents, although with limited indications. These pharmaceuticals include two synthetic Δ9-THC formulations, dronabinol (Marinol®, Syndros®) and nabilone (Cesamet™) and one CBD extract (Epidiolex®). This scenario urges the advance in the comprehensive understanding of the potential harmful consequences and therapeutic applications of Cannabis, Δ9-THC, and CBD. This is highly relevant because evidence indicates that phytocannabinoid properties are complex. For instance, acute Cannabis produces dose-dependent, biphasic effects on anxiety reducing it at low doses but increasing anxiety levels at high doses4,5. Furthermore, although Δ9-THC has a recognized antiemetic effect that resulted in one of the approved cannabinoid therapeutic uses, the chronic consumption of high Cannabis doses induces the Cannabis hyperemesis syndrome, characterized by persistent nausea and vomiting6. This complexity makes clear the need of continued research on the mechanisms of action underlying cannabinoid therapeutic effects to assure both their safety and effectiveness. This review examines the current state of knowledge about the opposed perspectives of the effects of Cannabis and its active principles, Δ9-THC and CBD: Their abuse potential and therapeutic use, two sides of the same coin.
THE PLANT
Marijuana is a preparation from the plant C. sativa. Its origin appears to be Central Asia, with a later spread to Africa, followed by Europe and, finally to the Americas7 (Fig. 1).
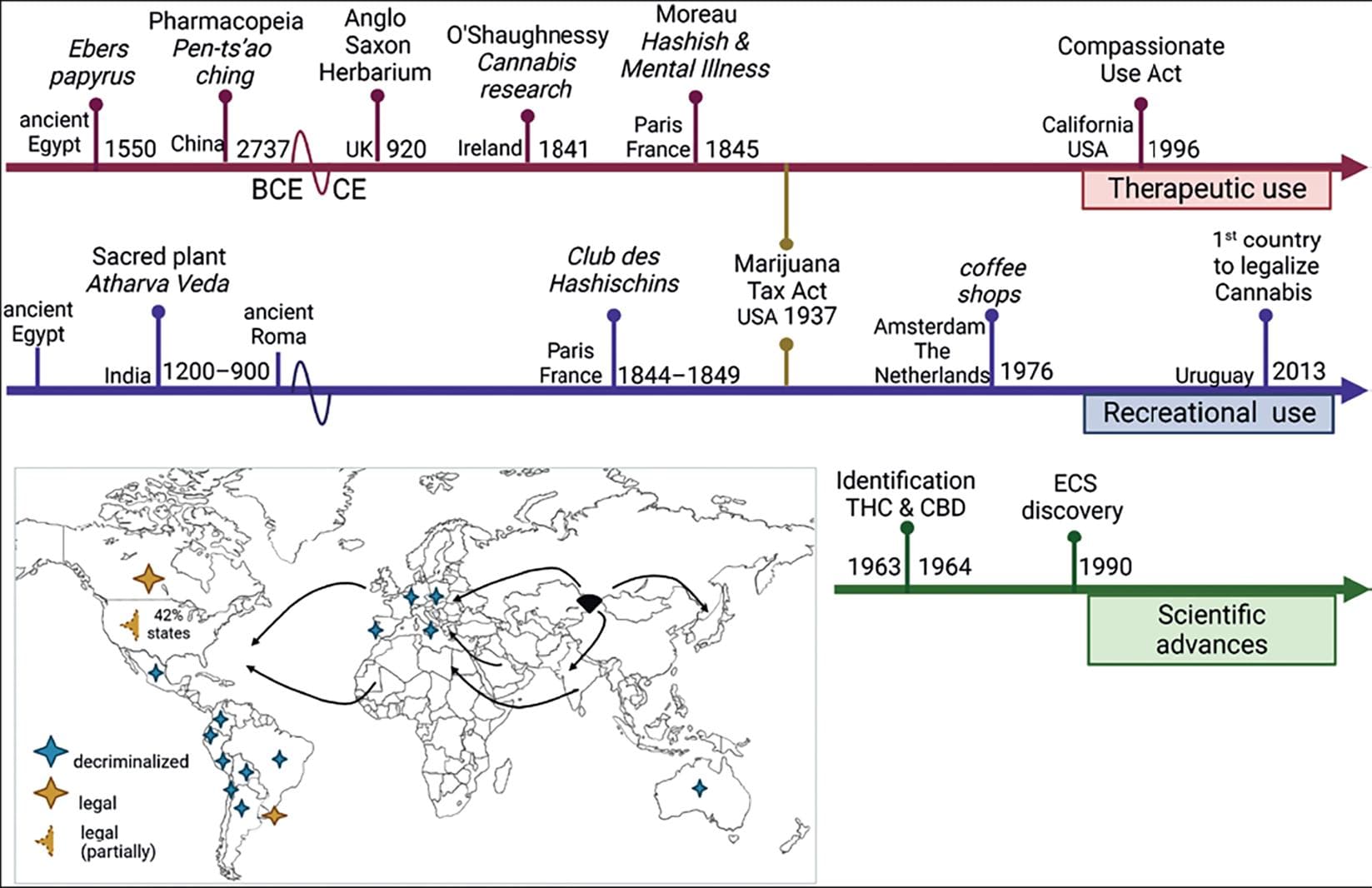
Figure 1. Cannabis history. Timeline tracing some historical milestones about the therapeutical (red) and recreational (blue) use of marijuana; the years of the breakthrough that led to the scientific study of the plant are also depicted. In the map, the arrows represent how it is believed that the plant was distributed from Asia to the rest of the world, and the colored stars illustrate the countries that legalized and decriminalized medical and non-medical uses of the plant (adapted from Pisanti and Bifulco7). Created with BioRender.com.
The genus Cannabis includes two main species: Cannabis indica and C. sativa; however, a morphological or chemical distinction between these species is difficult to make8. Therefore, the designation C. sativa is considered suitable for all plants of this genus.
C. sativa is an annual herbaceous flowering plant that can grow up to 5 m. It is a dioecious plant, meaning that there are male plants that create pollen sacs and female plants which develop inflorescences consisting of several individual bunches of flowers covered by trichome glands containing resin (Fig. 2). C. sativa grows better in template climates, although indoor-controlled cultivation is more common in recent times. It is a highly adaptable plant, explaining why its cultivation is widely expanded.
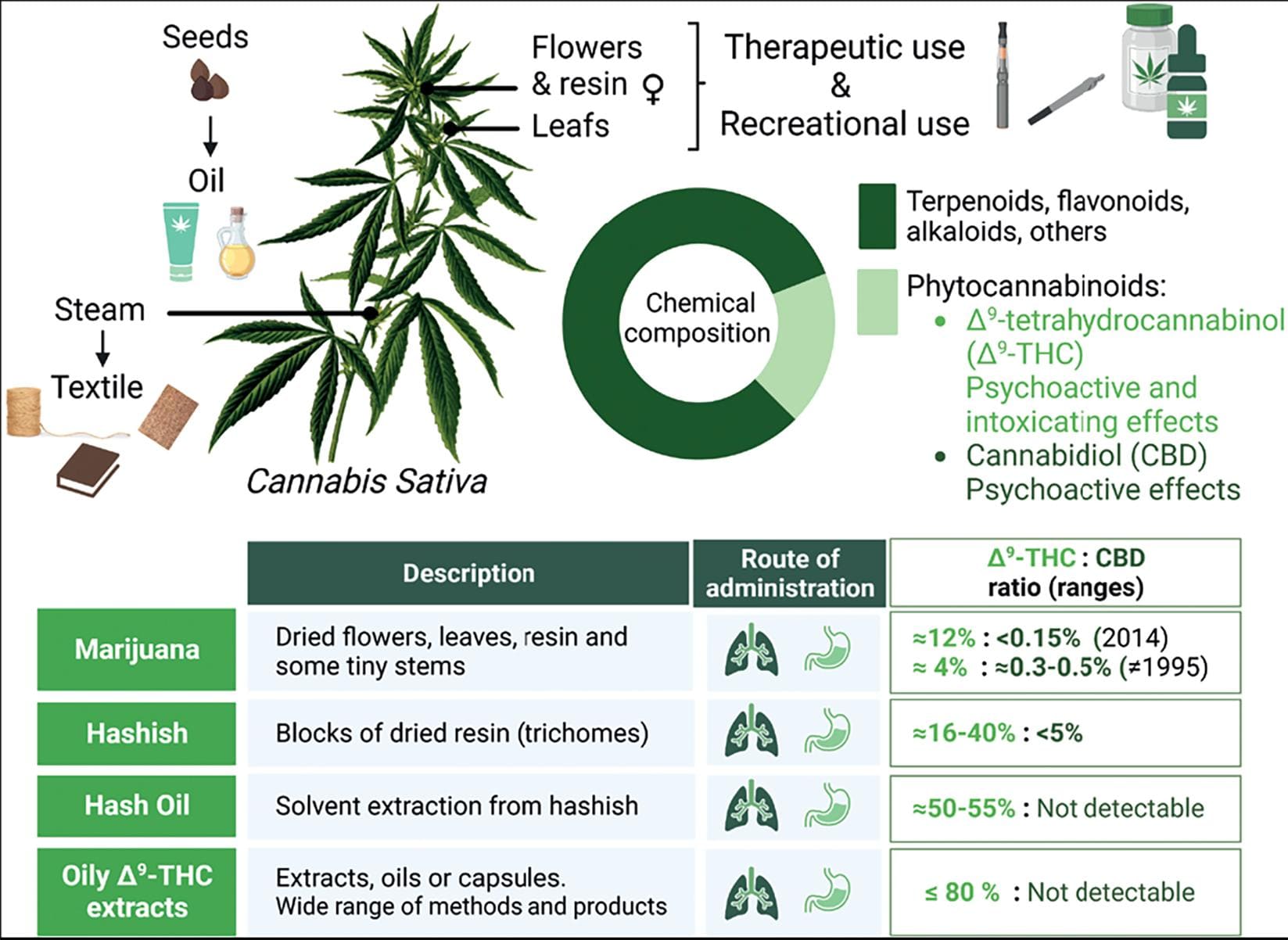
Figure 2. Cannabis sativa plant. From the family Cannabaceae, genus Cannabis, species sativa (Linnaeus, 1973). C. sativa is a versatile plant with multipurpose use. The fibers obtained from the stems, known as hemp, are durable and have been used in the textile and paper industry. The oil from the seeds is used in the cosmetic and food industry. The leaves, the flowers of the female plant, and the resin are used as preparations for its consumption. The plant's chemical composition can be divided into two main groups: phytocannabinoids, compounds exclusive of this plant including Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD), and other constituents such as terpenoids and flavonoids, among others. Phytocannabinoids are more abundant in the plant's resin, flowers, and leaves. The table shows the main Cannabis preparations that are offered. Marijuana is also known as mota, weed, ganja, pot, dope, and Maryjane, among other street names. Hashish is also known as just hash. Oily Δ9-THC, such as butane honey oil (BHO), can be obtained by different processes. The products derived from these highly efficient extractions receive a variety of names, such as shatter, wax, and dab8-10. Created with BioRender.com.
C. sativa contains approximately 500 compounds; around 100 are exclusive to the plant and are called phytocannabinoids, a term describing plant-derived cannabinoids. These compounds can be distinguished from synthetic cannabinoid molecules produced with therapeutic purposes (e.g., dronabinol). Synthetic cannabinoids also refers to the illicit molecules belonging to the new psychoactive substance group, recognized as a public health concern (e.g., "spice"). Phytocannabinoids are also distinct from those produced by living organisms known as endocannabinoids (eCBs) or endogenous cannabinoids (e.g., anandamide) (Fig. 3).
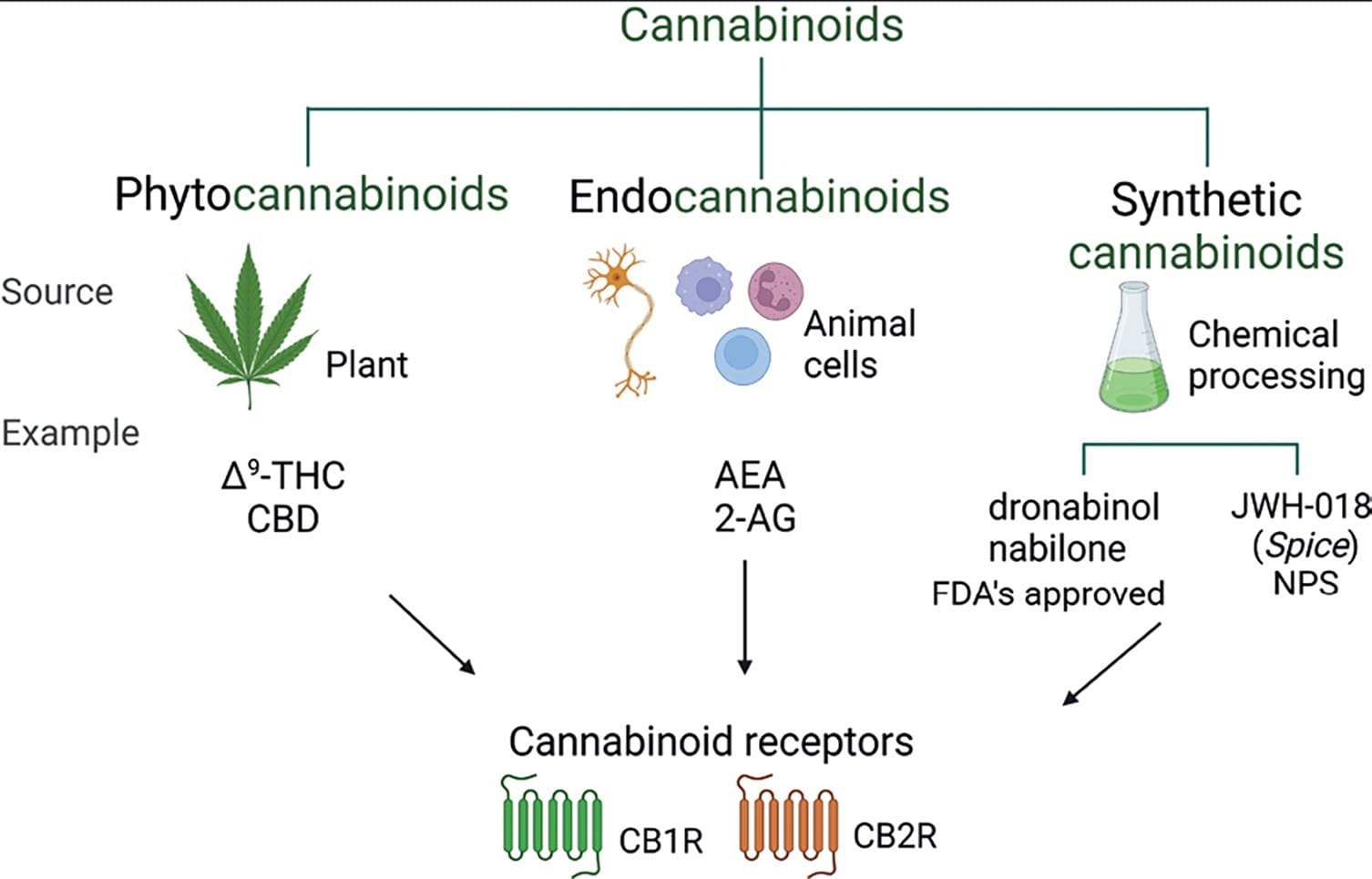
Figure 3. Cannabinoids. The term cannabinoids refers to molecules that, regardless of their origin, have the ability to interact with the cannabinoid receptors (CB1R and CB2R). Phytocannabinoids allude to the constituents of the C. sativa plant, such as Δ9-THC and CBD. Endocannabinoids is a term that designates those molecules synthetized by animal cells. Synthetic cannabinoids are those molecules produced in laboratories, as the name implies. For the synthetic cannabinoids, a distinction has to be made between those that are FDA approved to be used as therapeutic agents, such as dronabinol and nabilone, and those non-regulated due to its toxic effects that belong to the new psychoactive substances (NPSs) group of misused drugs, such as the JWH-018 found in products like Spice. Created with BioRender.com.
Δ9-THC and CBD are the major components of C. sativa, and both modify brain and body functions. Δ9-THC accounts for the intoxicating properties of Cannabis preparations, while CBD does not induce intoxication but exerts several psychoactive effects. The content of phytocannabinoids in the extracts of C. sativa is highly variable. The Cannabis plant has been semi-domesticated, and its Δ9-THC content increased deliberately, making Cannabis preparations with a high Δ9-THC content and a lower CBD content available9. Δ9-THC interacts directly, while CBD interacts indirectly with the ECS to exert their effects.
THE ENDOCANNABINOID SYSTEM
Δ9-THC effects are mediated by its ability to bind and activate cannabinoid receptors. These receptors are part of the ECS that encompasses cannabinoid receptors 1 and 2 (CB1R and CB2R), their endogenous ligands (eCBs), and the enzymes that participate in eCB biosynthesis and inactivation (Fig. 4). CB1R is expressed primarily in the brain11 and moderately in peripheral tissues, whereas CB2R is mainly expressed in the periphery. In the brain, CB2R is mostly present in microglia, particularly following inflammation, or injury12. Although, CB2R has been recently identified in neurons in discrete brain areas13. The endogenous ligands of these receptors are the eCBs, of which AEA and 2-AG, both derivatives of arachidonic acid, are the most studied. The ECS also includes the enzymes responsible for the synthesis and metabolism of AEA and 2-AG (Fig. 4).
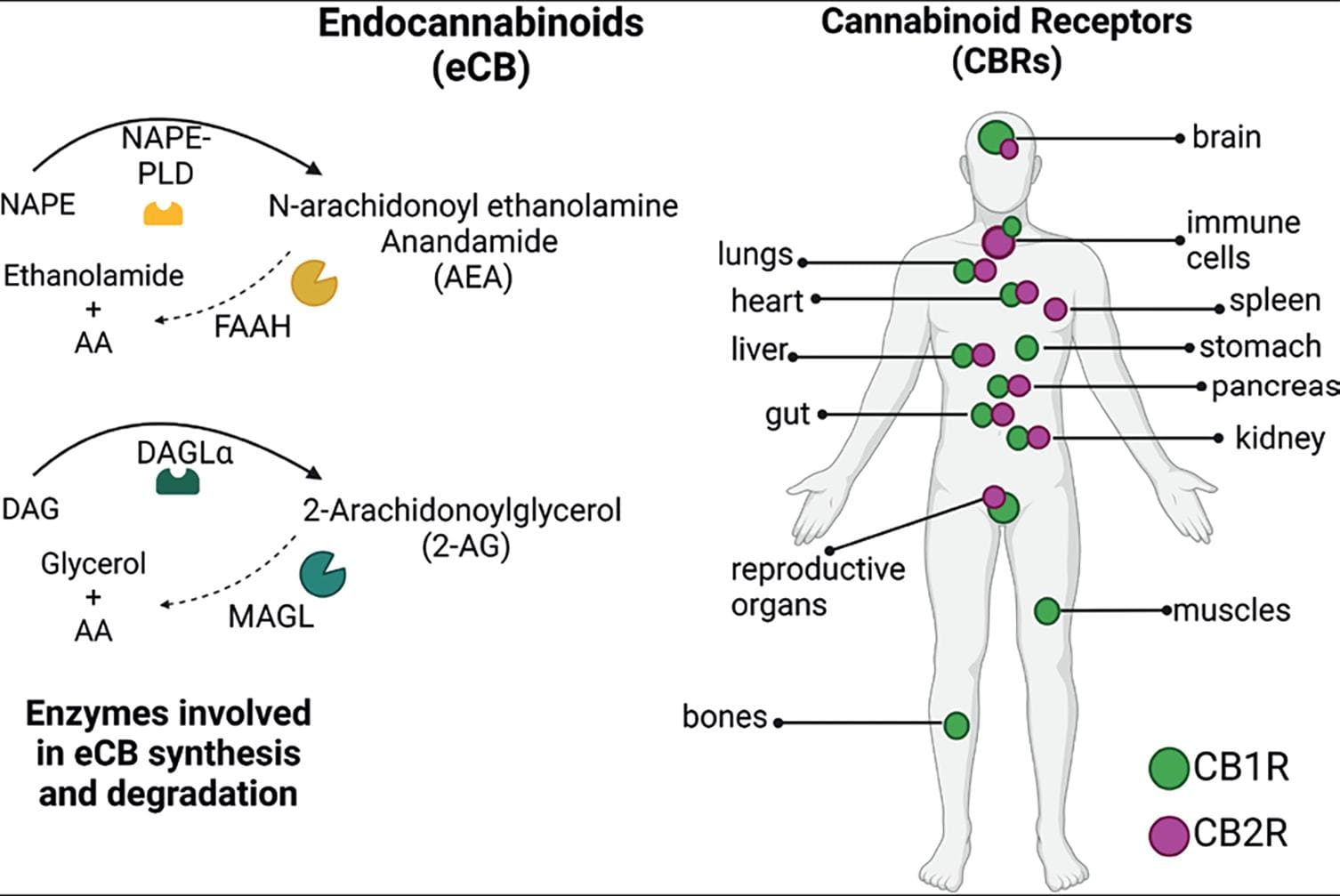
Figure 4. The endocannabinoid system. This system is present throughout the body and is composed of the cannabinoid receptors (CB1R and CB2R), their endogenous ligands (lipid-derived signaling molecules), of which the most studied and best characterized are N-arachidonoylethanolamine (anandamide, AEA) and 2-arachidonoylglycerol (2-AG), and the enzymes involved in AEA and 2-AG synthesis [N-acyl-phosphatidylethanolamine specific phospholipase D (NAPE-PLD) and diacylglycerol lipase α (DAGLα), respectively], and in their degradation [the fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), respectively]2,14. Created with BioRender.com.
Unlike other neurotransmitters, eCBs are not stored in synaptic vesicles but are synthesized and released "on demand;" this means that specific stimuli, like increased neuronal activity, trigger their production. In the brain, eCBs act as retrograde transmitters, that is, they are synthesized and released from the postsynaptic neuron, travel backward through the synaptic cleft, and bind to CB1R located on presynaptic axon terminals. CB1Rs are metabotropic receptors coupled to inhibitory Gi/o proteins, which activation hyperpolarizes the neuron, inhibiting the release of other neurotransmitters (Fig. 5). The ECS seems to play a role in many physiological activities and pathological conditions14, and it mediates the effects of Cannabis as a drug of abuse.
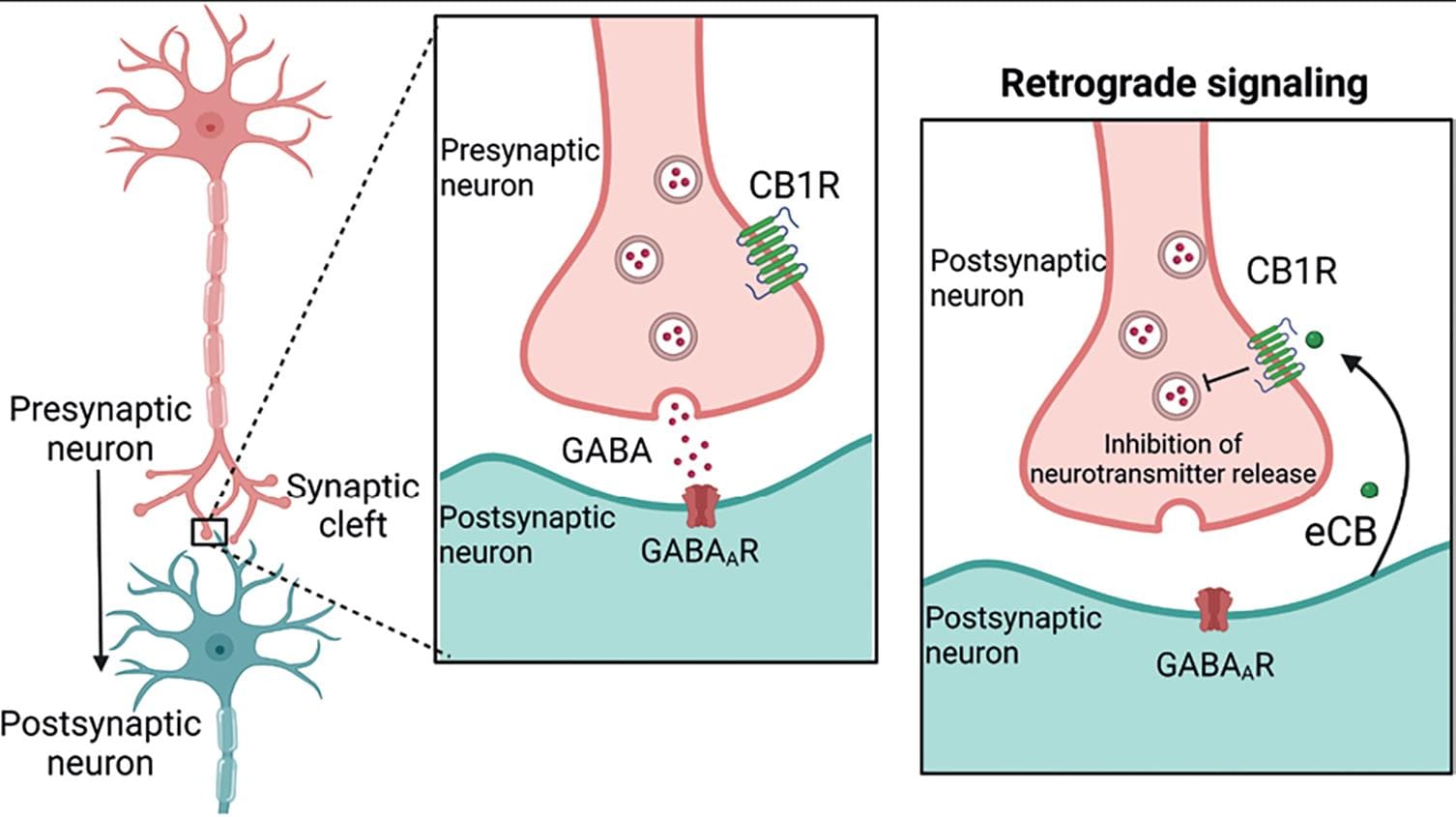
Figure 5. Retrograde signaling. In the brain, endocannabinoids (eCBs) are retrograde messengers, that is, they are synthesized and released by the postsynaptic neuron and act at CB1R located at the nerve endings of presynaptic neurons. CB1R activation leads to neuronal hyperpolarization and the inhibition of neurotransmitter release from the presynaptic terminal. This mechanism accounts for its neuromodulatory role at the synapsis2,14. Created with BioRender.com.
THE NON-MEDICAL USE OF CANNABIS
In 2020, the United Nations estimated that 209 million persons, or 4.2% of the global adult population, had consumed Cannabis in the previous year15. Strictly, "marijuana" refers to the crude mixture of dried and crumbled leaves, small stems, and flowering tops usually smoked in hand-rolled cigarettes (joints), or water pipes ("bongs"). However, Cannabis can be offered in different preparations. Hashish is a pure resin preparation with high cannabinoid content. With even more potency, new preparations such as butane hash oil or wax are also available. Extracts of the plant Cannabis loaded into cartridges for vaping also exist10; some of the characteristics of the different preparations are specified in table of figure 2. Countries where Cannabis use is regulated, such as specific states from the United States, Canada, and Uruguay commercialize diverse Cannabis products classified according to their chemotype, that is, the proportion of different phytocannabinoids (mostly Δ9-THC and CBD) contained.
Δ9-THC pharmacokinetics
The two most common methods of Δ9-THC administration are inhalation, through smoking or vaporization, and ingestion of edible Cannabis preparations. Smoking is the more efficient route to experience Δ9-THC psychotropic effects, which start soon due the lungs' large and highly vascularized absorption surface and can last around 3 h. After reaching peak levels, plasma Δ9-THC concentrations decline due to liver metabolism and drug accumulation in the body fat. When the preparation is consumed orally, its effects begin to be experienced at least half an hour later, due to the prolonged but poor Δ9-THC absorption by the gastrointestinal tract, increasing the probability that the consumer takes a second dose and thus becoming exposed to high Δ9-THC concentrations. These high concentrations often trigger adverse effects such as panic attacks.
Δ9-THC is metabolized in the liver producing nearly 100 different metabolites, of which 11-OH-THC and THC-COOH are the major ones found in humans. Δ9-THC accumulates largely in body fat, which serves as a long-term storage site for the drug. This characteristic explains Δ9-THC large elimination half-life16. Details of Δ9-THC pharmacokinetic parameters are shown in figure 6.
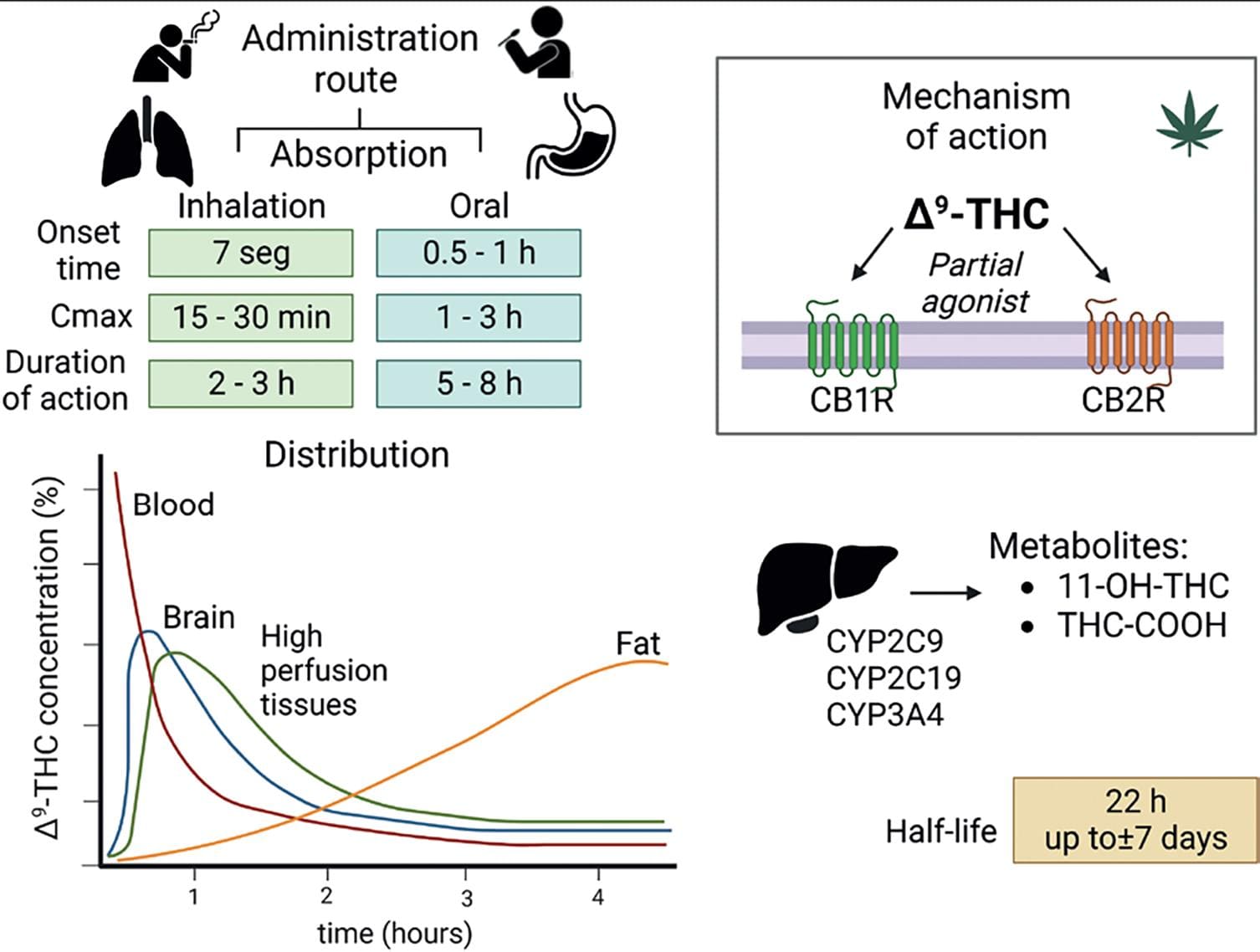
Figure 6. Δ9-THC pharmacology. Pharmacokinetic parameters related to drug absorption vary according to the route of administration. The graph depicts the distribution of the Δ9-THC molecule in different tissues. As seen, tissues with high perfusion, including the brain, show an initial increase in Δ9-THC concentration followed by a decrease. By contrast, Δ9-THC concentration in the fat shows a constant increase, an effect associated with the lipophilic nature of Δ9-THC and contributing to its prolonged half-life16. Hepatic metabolism generates 11-hydroxy-Δ9-tetrahydrocannabinol (11-OH-THC) and 11-nor-9-carboxy-THC-Δ9-tetrahydrocannabinol (THC-COOH). 11-OH-COOH is a psychotropic metabolite that is equipotent to Δ9-THC. THC-COOH, in contrast, is a non-psychotropic metabolite. Δ9-THC is a partial agonist of CB1R and CB2R16. Created with BioRender.com.
Δ9-THC mechanism of action
Δ9-THC is a partial agonist of CB1R and CB2R. Its effects have been described to be mediated by CB1R activation, though the participation of CB2R is beginning to be investigated. In the brain, CB1R is expressed in cortical areas involved in higher cognitive functions, in midbrain regions associated with motor control and reward, and in hindbrain regions controlling motor and sensory functions of the autonomic nervous system (Fig. 7).
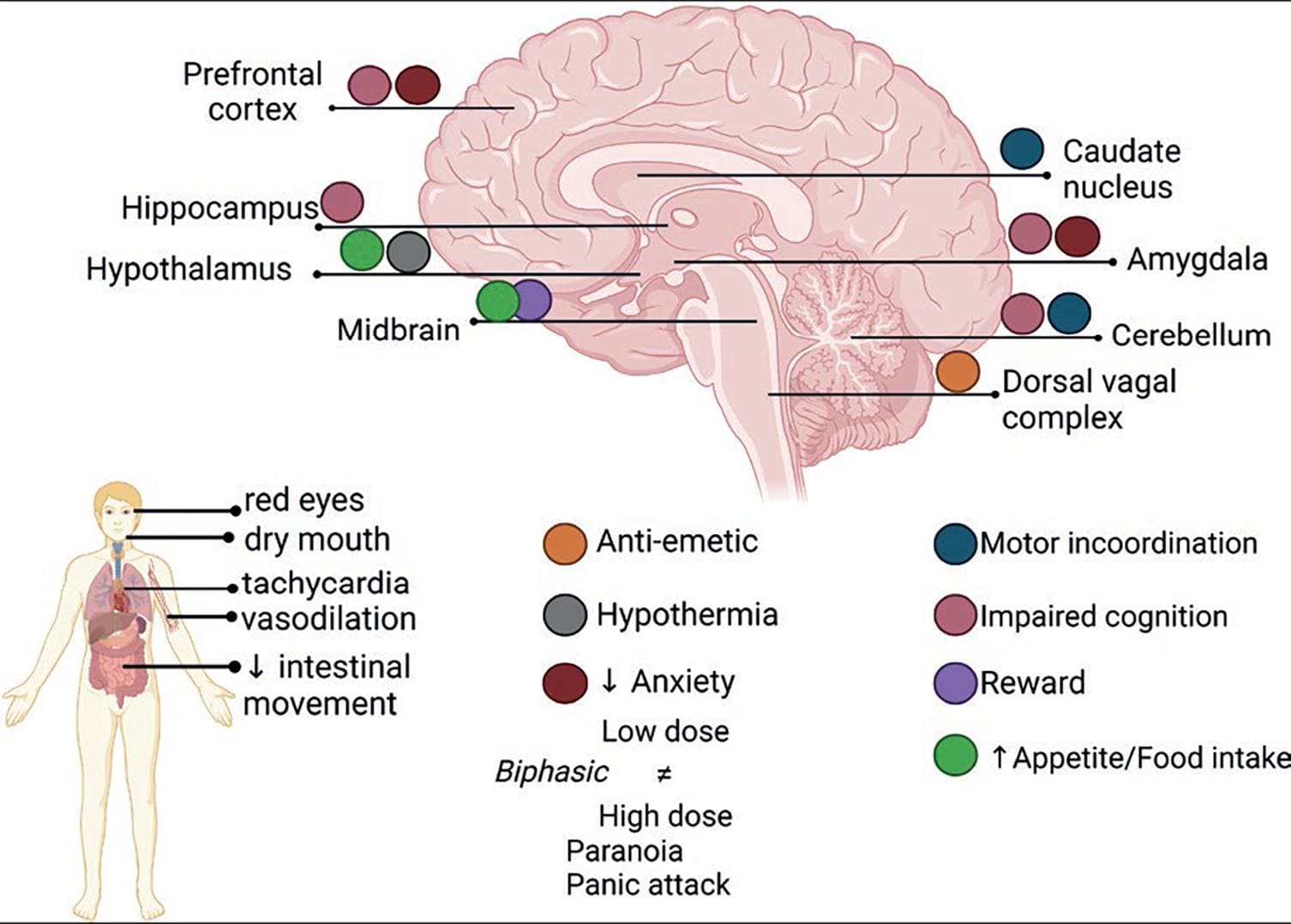
Figure 7. Δ9-THC acute effects. Illustration of the complex cluster of effects induced by marijuana or any other preparation of the C. sativa plant. Δ9-THC exerts its central effects by activating CB1R in different brain areas31. Created with BioRender.com.
Cannabis acute effects
In rodents, Δ9-THC and cannabinoid receptor agonists typically induce a tetrad of effects: Analgesia, hypothermia, catalepsy (lack of voluntary movement), and hypoactivity. This tetrad helps identifying the cannabimimetic activity of drugs. In humans, the effects of Cannabis depend on several factors, including the user's experience and possible tolerance, the expected effects and mental state, the environment (set and setting), and pharmacological factors like the administration route, which is highly relevant, especially in terms of the onset and duration of the effects. The Δ9-THC content in Cannabis preparations, which is highly variable, is a determinant factor for its effects.
Cannabis causes, depending on the dose, complex significant effects on the human body and mind, altering sensory perceptions, mood, cognitive abilities, motor coordination, and the sense of self and time. Acute Cannabis use is associated with subjective symptoms of mild euphoria, relaxation, continuous laughter and talkativeness, sedation, lethargy, intensification of ordinary sensory experiences, and perceptual distortion, for example, time perception, social withdrawal, and increased appetite and food consumption known as "the munchies." Physical signs include conjunctival hyperemia (red eyes) and ptosis, dry mouth, increased heart rate, mild increase in blood pressure, and orthostatic hypotension. The cluster of subjective and physiological effects induced by Cannabis intake is known as the "high." Other central nervous system effects are alteration of psychomotor functioning and impairment of cognitive tasks. Table 1 summarizes the main effects of Δ9-THC and their neurobiological bases, which are illustrated in figure 7.
Table 1. Summary of the acute effects of Δ9-THC
| System or response | Human effect | Animal models | Neurobiology | References |
|---|---|---|---|---|
| Ocular | Conjunctival hyperemia (red eyed) | – | vasodilation → ↓ BP ↑ blood flow in the eyeball = red appearance | 17 |
| ↓ IOP /≈ 4 h THR: glaucoma | ↓ IOP in rat model of glaucoma | CB1R ciliary processes: ↓formation of aqueous humor (↓ optic nerve damage) | 18 | |
| CV | Biphasic effects:
Variable changes in BP: Vasodilatation and ↓ BP, mostly diastolic |
Anesthetized animals: ↓HR and
↓BP Conscious animals: Bradycardia, ↓HR and hypotension in some animals. |
Complex hemodynamic effects CB1R-mediated | 19 |
| Orthostatic hypotension ▾ doses: dizziness | Postural (supine) hypotension→peripheral vasodilation and dysregulation of the baroreflex | 20 | ||
| GI | Biphasic effects:
THR: anti-emetic |
Dose-dependent suppression of Lithium induced vomiting in shrews. | Interaction with CB1R and 5-HT3
receptor (allosteric inhibition) at the dorsal vagal complex,
specifically in the area postrema of the brainstem, which
mediates emesis. ▾ doses act peripherally. Activation of CB1R in GI and enteric nervous system could be involved as well. |
21 |
| Interaction with CB1R in nerve fibers and synapses throughout the gut wall and in the myenteric and submucosal plexuses of the enteric nervous system | 22 | |||
| Temperature | Hypothermia | Hypothermia | Thermoregulatory centers of the anterior hypothalamus | 23 |
| Analgesia | Limited (ethical issues) intradermal
capsaicin-induced pain:
THR: Analgesic |
↑ Of the pain threshold in
models of thermal pain (hot plate/tail
flick). Antinociceptive effect |
CB1R in brain and spinal cord integrative sites; nociceptive sensory neurons of the dorsal root ganglion and trigeminal ganglion, and immune cells. | 24 |
| Anxiety | Biphasic effects: | Biphasic:
CB1R KO mice: ↑increased anxiety-like behavior |
Anxiolytic effect: CB1R on cortical
glutamatergic neurons. Anxiogenic effect: CB1R on forebrain
GABAergic neurons. ECS: modulates the response to stress (HPA & SNS), reward (MSL & PFC), and their interactions. Brain areas: hippocampus, PFC, amygdala, hypothalamus |
4,5 |
| Food intake | ↑ feeding, even in a state of
satiety emphasis on palatable-dependent appetite THR: Anorexigenic SR141716A (rimonabant): 1st approved drug but later removed from the market. |
Bimodal feeding
response: Activation of CB1R → ↑↑↑ feeding (despite satiety) Blockade of CB1R: ↓ food intake CB1R KO: lean phenotype, resistant to diet-induced obesity |
Food intake is a complex behavior,
CB1R are expressed in several brain regions and circuits
regulating different aspects like feeding behavior, intake
regulation, and satiety. Δ9-THC might act at:
The ECS has been recognized as critical for energy homeostasis and food intake regulation. |
25,26 |
| Motor | 7% Δ9-THC: impairs
complex psychomotor performance. 11% Δ9-THC: alters movement speed and balance |
↓spontaneous locomotor activity: catalepsy | High-density CBR in the caudate nucleus and cerebellum. | 27 |
| Cognitive | Mild-to-moderate
performance impairment of several functions:
Learning and (episodic) memory, |
CBRs expressed in brain areas involved in cognitive processes, such as PFC, hippocampus, and amygdala. | 28 | |
| Altered time perception: subjective perception of time = overestimation of the passage of time | Temporal estimation is processed in the cerebellum, a brain structure with high CB1R density. | 29 | ||
| Driving abilities are significantly impaired, although modestly | Driving-related neurobehavioral skills and driving performance are integrated in diverse brain areas where CB1R is expressed. | 30 | ||
BP: blood pressure; CBRs cannabinoid receptors; CNS: central nervous system; CV: cardiovascular; ECS: endocannabinoid system; GI: gastrointestinal; HPA hypothalamic–pituitary–adrenal axis; HR: heart rate; IOP: intraocular pressure; MJ: marijuana; MSL: mesolimbic system; PFC: prefrontal cortex; SNS: sympathetic nervous system; THR: Therapeutic relevance; Δ9-THC: Δ9-tetrahydrocannabinol.
▴: high dose ▾ low dose, ▾ ▴ medium dose. ↑ increased effect, ↓ decreased effect.
The consumption of high Δ9-THC doses may precipitate panic attacks or persistent paranoia as clinically significant adverse effects. Notwithstanding, a lethal Δ9-THC dose or deaths due to Cannabis overdose have not been reported, which might be related to the absence of CB1R expression in the brainstem.
Remarkably, Δ9-THC produces dose-dependent, biphasic effects, a feature shared by other phytocannabinoids such as CBD, eCBs, and synthetic cannabinoids. Thus, low and high doses of these compounds may exert opposite effects, or the effects of low doses get lost at higher doses32. The search for a state of mental relaxation and well-being is believed to be one of the factors driving the widespread consumption of Cannabis5.
Cannabis rewarding effect
As with other misused drugs, the rewarding effects of acute Δ9-THC intake are related to increases in dopaminergic neuron activity and dopamine release at the mesolimbic system (brain reward system)33. Since CB1Rs are not expressed on mesolimbic dopamine neurons, the Δ9-THC-induced increase in dopaminergic activity is likely produced indirectly by interacting with the CB1R located on the axon terminals of GABAergic and glutamatergic inputs that modulate the activity of midbrain dopamine neurons34 (Fig. 8). Neuroimage studies further demonstrated increased dopamine neuron activation in the human limbic striatum in response to acute Δ9-THC35. In contrast, long-term use of Δ9-THC is associated with a blunted dopamine system's activity, which is associated with reduced motivation and negative emotions33.
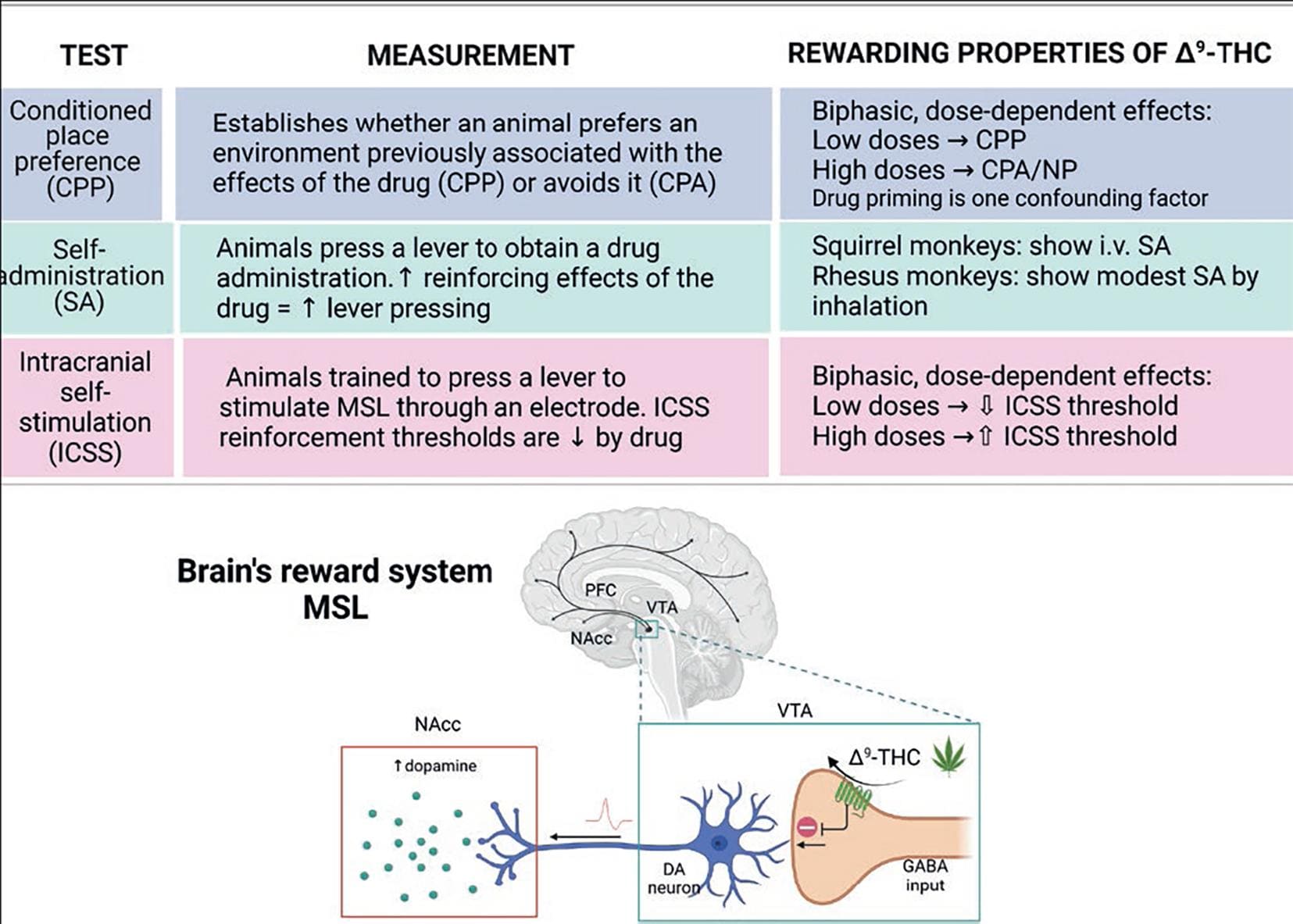
Figure 8. Rewarding properties of Δ9-THC. The table summarizes the animal models used to evaluate the rewarding properties of drugs of abuse. Like all other misused drugs, Δ9-THC increases dopamine (DA) levels in the nucleus accumbens (NAcc) of the brain's reward system, constituted by the mesocortical and mesolimbic (MSL) dopaminergic pathways. Dopaminergic neurons' activity is under a tonic inhibitory control of GABAergic inputs. Δ9-THC inhibits GABA release by activating the CB1R expressed on their nerve endings, thereby eliminating the inhibitory tone. As a result, DA neuron activity increases, augmenting DA release at the NAcc34,36-38. Created with BioRender.com.
Demonstrating the rewarding properties of Δ9-THC using animal models has been challenging because animal responses to Δ9-THC are less evident than those elicited by other misused drugs, such as cocaine or heroin. Thus, in the self-administration (SA) paradigm, Δ9-THC induces a modest response, and this only in non-human primates. In the conditioned place preference model (CPP) and the intracranial self-stimulation (ICSS) protocol, Δ9-THC exhibits a biphasic profile; whereas low doses induce CPP and decrease the ICSS threshold, high doses induce conditioned place aversion and increase the ICSS threshold. The table in figure 9 summarizes these results and briefly describes the models36-38.
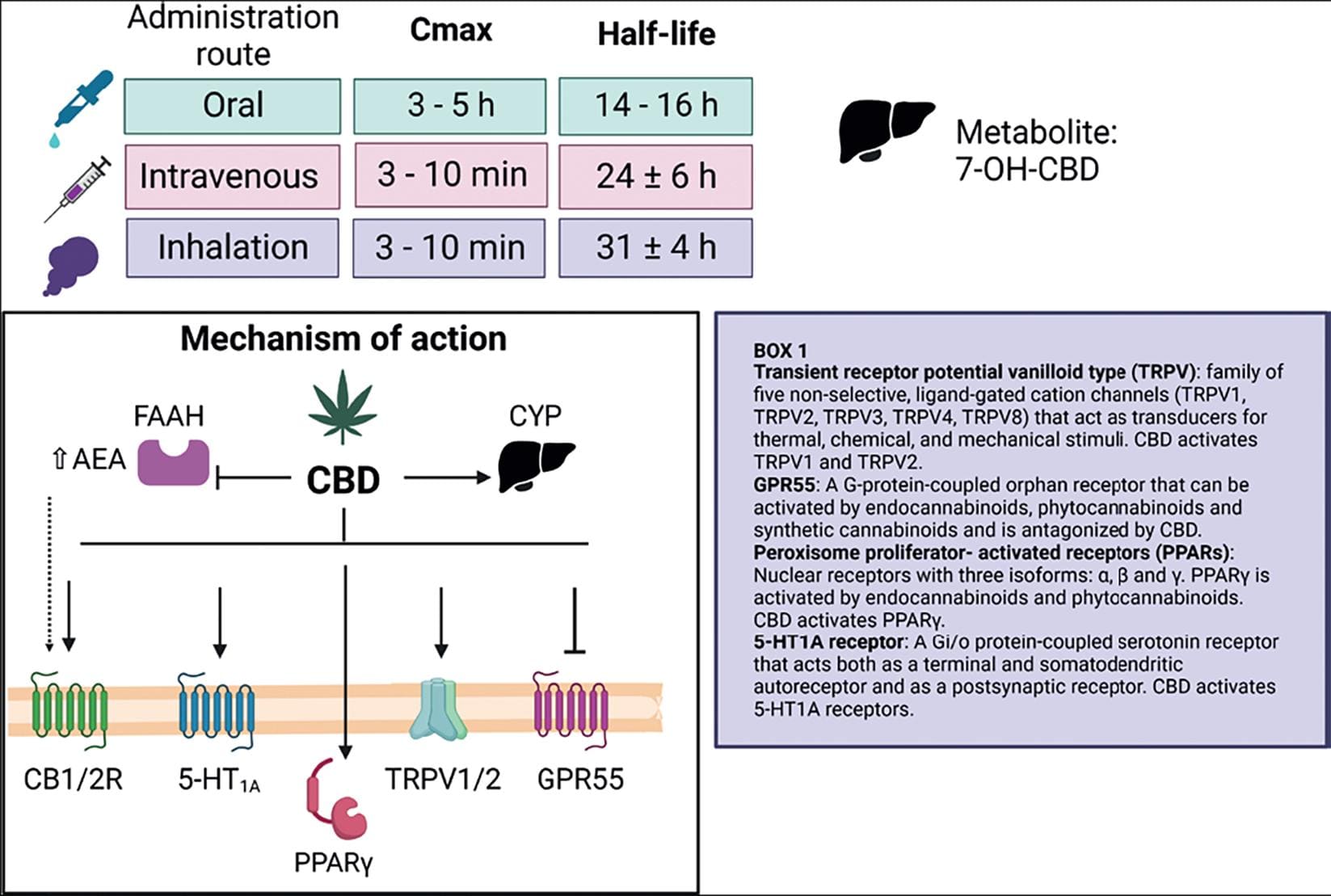
Figure 9. CBD pharmacology. CBD pharmacokinetic parameters related to drug absorption vary according to the route of administration. Hepatic metabolism generates 7-OH-CBD. The main CBD molecular targets are CB1R and CB2R, 5-HT1A and GPR55 metabotropic receptors, TRPV1 and TRPV2 channels, and the PPARγ nuclear receptor. CBD can inhibit the AEA-degrading enzyme FAAH, thereby increasing its concentrations. CBD acts as an agonist (↓) or as an antagonist (⊥) on these targets. Box 1 includes a brief description of CBD molecular targets16. Created with BioRender.com.
Effects of Cannabis use and Cannabis use disorder
Frequent Cannabis use has deleterious health effects (Table 2) and has been associated with several psychiatric conditions (Table 3). Two syndromes produced by heavy and chronic marijuana use have also been described: The Cannabis hyperemesis syndrome (Table 2) and the amotivational syndrome (Table 3). Although the latter has been a matter of debate, it is clear that the abuse of marijuana intake can lead to adverse consequences in cognitive areas and mental health, including the development of Cannabis Use Disorder (CUD).
Table 2. Summary of adverse effects of chronic Cannabis consumption
| Adverse effect | Description | Reference |
|---|---|---|
| Respiratory symptoms | Smoking or vaping CN may lead to respiratory symptoms and lung injury due to the exposure to combustion products and/or other harmful chemicals. | 39 |
| Smoking CN (> 1×/week for ≥ 1 year) is associated with respiratory symptoms: cough, sputum production, and wheezing. | ||
| Cardiovascular | Smoking CN can be as harmful as tobacco smoke. | 40 |
| Dysregulation of the ECS has been implicated in several CV pathologies. Activation of CB1R (CNS and CVS) facilitates the development of cardiometabolic disease. | ||
| Cessation of use → ↑BP and HR in heavy CN users. | ||
| Highly potent CN and synthetic cannabinoids: associated with more serious adverse CV events (↑ risk of cardiac arrhythmias, myocardial infarction, and angina) | ||
| Heavy CN users: ↓ cerebral blood flow (↑ on the cessation of use) | ||
| Cannabinoid hyperemesis syndrome | Functional gut-brain axis disorder characterized by cyclic episodes of nausea and vomiting and frequent hot bathing (learned behavior to reduce the discomfort), worsened by Cannabis intake. The paroxysms are intense and incapacitating. Patients vomit profusely, often without warning (up to 5 times/h). Paradoxical effects on GI tract and CNS | 6,21 |
| Gynecological and obstetric complications, and adverse male sexual health effects | Chronic CN use: ↓ human reproductive potential | 41 |
| ♀ Fertility. Menstrual cycle disruption: ovulation delay and cyclicity inhibition. ♂ ejaculatory problems, sperm count and motility reduction, loss of libido and impotence | ||
| CN use in pregnancy → impairs embryo implantation. | ||
| Adverse neonatal outcomes: low birth weight, preterm birth, admission to neonatal intensive care, and small size for gestational age. Prenatal CN exposure influences brain development and may have long-lasting effects on cognitive functions. | ||
| Cognitive consequences | Mixed results (difficulties related with quantification of the retrospective CN consumption). | 42,43 |
| Mild residual cognition impairing effects: processing speed, attention, learning capabilities; short-term memory and verbal episodic memory. Impaired executive functions: working memory. | ||
| Cognitive deficits seem to be linked with the early onset of CN use. Preclinical data support the implication of CN use during important stages of neurodevelopment (adolescence). |
BP: blood pressure; CN: Cannabis; CNS: central nervous system; CV: cardiovascular; CVS: cardiovascular system; ECS: endocannabinoid system; GI: gastrointestinal; HR: heart rate.
Table 3. Summary of psychiatric disorders associated with Cannabis use and dual diagnoses with Cannabis use disorder
| Syndrome | Characteristics | Reference |
|---|---|---|
| Amotivational syndrome | Heavy chronic CN users are more likely to experience apathy and passivity, leading to loss in productivity and diminution or absence of drive to engage in typically rewarding activities. The diagnosis remains uncertain. | 44 |
| Psychosis and schizophrenia | Daily and/or high potency CN use increases the odds (≈5 times) of psychotic disorder/episode (vs. no CN users). | |
| CN use: risk factor for early onset of schizophrenia in people with predisposition and/or exacerbation of the psychotic symptoms already present. | 45,46 | |
| Depression | Bidirectional relationship. CN use during adolescence and/or heavy CN use is associated with a moderately increased risk of developing MDD or other depressive disorders. | |
| CN use is associated with a worse prognosis in individuals with MDD. | 47,48 | |
| Anxiety | Bidirectional relationship. The odds of CUD among individuals with social anxiety disorder is higher (≈ 5 times) than among individuals without the disorder. | |
| At low doses, CN use can enhance the extinction rate and reduce anxiety responses in PTSD. | 49,50 | |
| ADHD | Childhood ADHD increases the chances of CN use and CUD. (Limited evidence). | 51 |
| BPD | Borderline traits contribute significantly to CN use and to develop CUD. (Limited evidence). | 52 |
ADHD: attention-deficit/hyperactivity disorder; BPD: borderline personality disorder; CN: Cannabis; CUD: Cannabis use disorder; MDD: major depressive disorder; SUD: substance use disorders; PTSD: post-traumatic stress disorder.
It has been estimated that ≈ 9% of chronic Cannabis users will develop a severe CUD according to DSM 5 criteria31, and this percentage increases when use begins before 18 years of age (≈ 16.6%). CUD is defined as the inability to stop consuming marijuana even when it is causing physical or psychological harm (ICD, WHO web). The DSM 5 criteria for CUD are presented in table 4. Pharmacological criteria for CUD are the appearance of tolerance to some of the Δ9-THC effects, including cardiovascular, cognitive, and physical effects53.
Table 4. DSM 5 criteria for CUD
CUD severity: mild CUD (2-3 symptoms); moderate CUD (4 or 5 symptoms) or severe CUD (> 6 symptoms). CUD: Cannabis use disorder; DSM: diagnostic and statistical manual of mental disorders.
Cannabis withdrawal (CW) is the manifestation of the development of physical dependence on Δ9-THC and emerges when a person stops its use. Because Δ9-THC accumulates in fat tissue and its clearance rate is slow, the CW signs and symptoms become evident not earlier than 1 week after interrupting marijuana consumption. CW can also be precipitated by CB1R antagonists. The CW syndrome includes anger, irritability, depression, restlessness, headache, loss of appetite, insomnia, and severe craving for marijuana. The complete list of symptoms of the CW syndrome is presented in table 4.
CANNABINOID THERAPEUTIC POTENTIAL
The most ancient record of the use of Cannabis for therapeutic purposes is the world's oldest pharmacopeia, the Sheng-nung Pen-ts'ao Ching (China, 2737 BC)54. However, traditional use of Cannabis preparations for treating medical conditions does not constitute valid evidence for the modern medical use of the plant. High-quality pharmacological studies in large, well-controlled clinical trials are required to ensure the efficacy and safety of the therapeutic use of the phytocannabinoids, isolated, or combined.
The characterization of the most abundant phytocannabinoids in the Cannabis plant, Δ9-THC and CBD, and the search for their mechanisms of action allowed the identification of the ECS. This system is distributed throughout the body and plays a key role in regulating many physiological processes and maintaining homeostatic balance. Therefore, the pharmacological modulation of physiological and pathological processes by cannabinoids appears therapeutically promising. The neuromodulatory role of the ECS is of particular interest since many neuropsychiatric conditions rely on neurochemical imbalances affecting the functioning of brain circuits.
Here, we review the available evidence for the suggested therapeutic applications of Δ9-THC and CBD.
Cannabidiol pharmacokinetics
CBD is one of the most abundant phytocannabinoids of C. sativa. Its acute administration by distinct routes does not produce significant toxic effects in humans across a wide range of concentrations and is well tolerated at doses up to 1500 mg/day, as well as with chronic use55. In general, CBD has a favorable safety profile56,57 and lacks abuse liability58, two important features to consider when proposing it as a therapeutic agent for treating pathological conditions.
However, CBD is a potent inhibitor of hepatic drug metabolism and acts both as an inhibitor and an inducer of several cytochrome P450 isoforms, properties that might affect the metabolism of other drugs in vivo (for a review on drug-drug interactions, see59).
CBD is a highly lipophilic compound with poor oral bioavailability15. In human studies, maximum CBD concentration is achieved 3-5 h after its ingestion in healthy adults. CBD has a half-life of 14.4-16. Six hours after its oral administration and, in the presence of a high-fat meal, CBD exposure time significantly increases (4-fold)60. Following intravenous dosing, the average CBD half-life is 24 ± 6 h and 31 ± 4 h after its inhalation; these two administration routes present similar pharmacokinetics, reaching peak plasma concentrations in 3-10 min and an average bioavailability of 31%15. CBD intake by the oral route is subjected to first-pass metabolism and can therefore be transformed by liver enzymes before reaching the gut61. Hepatic hydroxylation of CBD produces 7-OH-CBD, which undergoes mainly fecal but also urinary excretion16 (Fig. 9).
Cannabidiol mechanism of action
CBD has multiple molecular targets (Fig. 9) (62 for a comprehensive review). It has a low affinity for CB1R and CB2R but can interact with them at 1 μM concentrations and acts as an inverse agonist at CB2R. CBD is also an antagonist at GPR55 receptors and an agonist at TRPV1, TRPV2, PPARγ, and 5-HT1A receptors63 (Glossary of CBD molecular targets in the box of Fig. 9).
In addition, CBD blocks AEA uptake and inhibits its enzymatic hydrolysis, thereby increasing AEA concentrations64, and antagonizes Δ9-THC effects by interacting with non-cannabinoid receptors, like GPR5565. CBD can also increase Δ9-THC potency through pharmacokinetic or pharmacodynamic interactions66.
Cannabis-based prescription drugs
Among the prescription drugs derived from Cannabis approved for medical use, there is only one containing Δ9-THC and CBD in a 1:1 ratio: Sativex®. Besides, two Δ9-THC synthetic formulations, Marinol® and Cesamet® and one CBD extract, Epidiolex®, have also been approved and are currently available in the market. These formulations are being used in clinical trials aimed to support their putative therapeutic actions and there is continued search for new cannabinoid therapeutic uses.
It is important to highlight that a significant number of unregulated cannabinoid products are being offered in the market, mainly CBD, in different formulations (capsules, oils, tinctures, creams, e-liquids, wax for vaporization, and dietary supplements), in many of which the advertised CBD content and purity (some of them contaminated with Δ9-THC) were found to be inaccurate when analyzed67. Still, CBD is consumed in a variety of over-the-counter products, various sold online, for the self-treatment of numerous conditions, for which clinical evidence is lacking or is not supported by robust controlled clinical trials. This makes it urgent to run high-quality clinical trials investigating the putative CBD usefulness for the treatment of those medical conditions that are advertised without sufficient scientific support.
Epidiolex® is a CBD product approved by the FDA for the treatment of seizures associated with Dravet syndrome, Lennox-Gastaut syndrome, and tuberous sclerosis complex. Evidence for all other CBD therapeutic effects is, at this moment, insufficient. Marinol® and Cesamet® prescription is strictly regulated for very specific medical conditions due to their abuse potential. The same occurs with Sativex®, but in this case, the therapeutic applications could be wider because, as mentioned earlier, CBD has been found to counteract some of the unwanted Δ9-THC-derived side effects and to complement its actions through molecular targets other than CB1R.
Δ9-THC potential therapeutic actions
The study of the ECS highlighted that activation of central CB1R is the primary mechanism mediating Δ9-THC psychoactive effects, as well as some of its potential therapeutic actions. However, the intoxicating properties of Δ9-THC limit its therapeutic use as an isolated agent. Notwithstanding, the FDA approved two Δ9-THC synthetic formulations (dronabinol and nabilone) as therapeutic agents to be used in specific conditions detailed in table 568-70.
Table 5. Summary of the therapeutic uses of Δ9-THC for different conditions, according to the report of Health Effects of Marijuana (HEM)69
| Condition | HEM | Approved Δ9-THC-based medications | Reference |
|---|---|---|---|
| Chemotherapy-induced nausea and vomiting | Conclusive evidence as effective antiemetics |
|
69 |
| Spasticity related with multiple sclerosis (MS) | Substantial evidence for improving patient-reported MS spasticity symptoms but limited evidence for an effect on clinician-measured spasticity | Nabiximols (Sativex®), approved by EMA (2010) and Canada. Cannabis sativa plant extract (containing Δ9-THC and CBD in near-equal amounts) oromucosal spray. Side-effects include dizziness, fatigue, blurred vision, vertigo, constipation, either appetite decrease or increase, and depression. Rare, but serious side effects include palpitations, changes in blood pressure, and hallucinations. | 68 |
| Chronic pain | Modest to substantial evidence for adults' effective treatment. Complex effects of cannabinoids-induced analgesia | Recent meta-analysis: Nabilone elicits a significant pain reduction in patients with neuropathy. Examples of ongoing clinical trials: Dronabinol after arthroscopic surgery (NCT05335252); Treatment of chronic pain with cannabidiol (CBD) and Delta-9-tetrahydrocannabinol (THC) (NCT03215940). | 70 |
| The synergism between THC and opioid medications is being explored, for example the clinical trial: Opioid-sparing effect of Dronabinol (NCT03766269) | |||
| Conditions with
moderate evidence: Sleep disorders: Improved short-term sleep outcomes in individuals with sleep disturbances associated with obstructive sleep apnea syndrome, fibromyalgia, chronic pain, and MS. | |||
Conditions with
limited evidence:
| |||
| Conditions with
insufficient evidence: Symptoms of irritable bowel syndrome, cancers, epilepsy, amyotrophic lateral sclerosis, Huntington's disease, Parkinson's disease, dystonia spasticity in patients with paralysis due to spinal cord injury, cancer-associated anorexia-cachexia syndrome, anorexia nervosa | |||
Examples of conditions
tested in currently ongoing clinical trials (FDA):
| |||
FDA: Federal Drug Administration (USA); EMA: European Medicines Agency; HEM: Health Effects OF Marijuana; MS: multiple sclerosis.
Cannabidiol potential therapeutic actions
The majority of the therapeutic properties ascribed to CBD are based either on the results of preclinical studies, using cell and animal models, or on the involvement of the identified molecular targets of CBD in different pathologies. Only a few clinical trials validate those putative therapeutic properties. Here, we summarize those clinical trials (Table 6) and present an overview of preclinical studies, which support potential CBD therapeutic actions or point to novel putative clinical applications (Table 7).
Table 6. Cannabidiol therapeutic effects
| Disorder | Studied population | Dose and route of administration | Therapeutic effects | Reference |
|---|---|---|---|---|
| Schizophrenia | Adult schizophrenics (n = 42) | 800 mg/day (4× 200 mg/day) for 4 weeks, oral (RDCT) | ↓ of psychotic symptoms like amisulpride but with less side effects | 71 |
| Schizophrenics (n = 88) | 1000 mg/kg/day for 6 weeks, oral (RDCT) | ↓ of positive psychotic symptoms | 72 | |
| Parkinson disease (PD) | Adult PD patients with psychotic symptoms (n = 6) on L-DOPA treatment | 150 – 400 mg/day for 4 weeks, oral (OLT) | Improvement of psychotic symptoms without affecting motor or cognitive functions | 60 |
| Adult PD patients (n = 21) | 75 or 300 mg/kg/day for 6 weeks, oral (RDCT) | 300 mg/kg CBD dose improved well-being and quality of life without affecting motor and general symptoms | 60 | |
| Refractory epilepsy | Patients aged 1-30 (n = 137), with severe, treatment-resistant childhood-onset seizures | from 2-5 mg/kg/day up to 25-50 mg/kg/day, oral (OLT) | ↓ in monthly seizure frequency | 60 |
| Children and young adults (n = 120) with Dravet syndrome | 20 mg/kg/day, oral (RDCT) | ↓ in monthly seizure frequency | 60 | |
| Patients aged 2-55 (n = 212) with Lennox-Gastaut syndrome | 10 or 20 mg/kg/day, oral (RDCT) | ↓ of drop seizures by both CBD doses | 60 | |
| Patients aged 2-55 (n = 171) with Lennox-Gastaut syndrome | 20 mg/kg/day, oral (RDCT) | ↓ in drop seizures | 60 | |
| Children (n = 77) and adults (n = 62) with various types of epilepsy | 5-50 mg/kg/day, oral (OLT) | Reduction in seizure severity and ↓ frequency | 60 | |
| Patients (n = 18) with Tuberous sclerosis complex | 50 mg/kg/day, oral (OLT) | ↓ in monthly seizure frequency | 60 | |
| Anxiety | Healthy adults (n = 47) | 150, 300 or 600 mg, oral (RDCT) | ↓ anxiety during simulated public speaking, only with the 300 mg dose | 60 |
| Undergraduate students with social phobia (n = 24) and healthy subjects (n = 12) | single 600 mg dose, oral (RDCT) | ↓ anxiety during simulated public speaking | 60 | |
| Anxiety | Healthy adults (n = 60) | 100, 300 and 900 mg, oral (RDCT) | ↓ subjective anxiety ratings during public speaking only at the 300 mg dose | 60 |
| Young adults aged 20-33 with generalized social anxiety (n = 10) | 400 mg, oral (RDCT) | ↓ anxiety on a Visual Analog Mood Scale | 60 | |
| Adolescents with social anxiety disorder (n = 37) | 300 mg/day/4 weeks (RDCT) | Improved social anxiety | 73 | |
| Fragile X syndrome patients, aged 6–17 y (n = 20) | 50 mg/day; 50 or 125 mg twice daily for 12 weeks, transdermal (OLT) | Clinical meaningful reductions in general anxiety and social avoidance | 74 |
Note: Reference 60 is a review containing the specific citations for the different studies summarized. RDCT: randomized, double-blind, controlled trial; OLT: open-label trial.
Table 7. Preclinical evidence for cannabidiol potential therapeutic applications
| Disorder | Evidence | Animal model | CBD dose | Effects | Reference |
| Schizophrenia | Reduction of psychotic-like symptoms | Apomorphine-induced stereotypy | 15-60 mg/kg | ↓ stereotyped behavior | 78 |
| haloperidol-induced catalepsy | 30-60 mg/kg | ↓ catalepsy | 79 | ||
| D-amphetamine-and ketamine-induced hyperlocomotion | 30-60 mg/kg | ↓ hyperlocomotion | 80 | ||
| MK-801-induced PPI disruption | 5 mg/kg | Reverses PPI disruption | 81 | ||
| Epilepsy | Anticonvulsant effects | Pentylenetetrazole model of generalized seizures | 100 mg/kg | ↓ incidence of severe seizures and mortality | 82 |
| Acute pilocarpine model of temporal lobe seizure | 1-100 mg/kg | ↓ % of animals showing severe seizures | 82 | ||
| Penicillin model of partial seizure | 10-100 mg/kg | ↑ seizure-induced mortality | |||
| Anxiety | Prevention of anxiogenic effects | chronic unpredictable stress (14 days) | 30 mg/kg/day after stress for 14 days | Blocked anxiogenic-like effects measured in the novelty suppression feeding and EPM; ↑ hippocampal neurogenesis and ↑ AEA levels | 83 |
| Disorder | Evidence | Animal model | CBD dose | Effects | Reference |
| Anxiety | foot shock stress applied 24 h before the light/dark test | 5 mg/kg acute and chronic administration (21 days) | Prevented anxiety-like responses (↓ time in the light box and ↑latency to enter the light box) to foot shock | 84 | |
| Anxiety reduction | EPM and novelty suppressed feeding | 30 mg/kg for 14 days | anxiolytic-like effects (↑ entries and time in open arms; ↓ latency to feed) in stressed animals | 85 | |
| Depression | Reduction of depression-like behavior | FST | 200 mg/kg | ↓ immobility but also ↓ locomotion | 86 |
| Increases in hedonic behavior and motivation | Wistar-Kyoto rat, genetic model of depression | 30 mg/kg | ↑ saccharin preference | 87 | |
| 45 mg/kg | ↑ exploration in the novel object test and locomotion | ||||
| Emesis | Reduction of vomiting and nausea | Lithium chloride – nicotine- or cisplatin-induced conditioned gaping reactions | 5 or 10 mg/kg | suppresses nausea-like (gaping) behavior | 88 |
| Lithium chloride – conditioned and unconditioned rejection reaction | 5 mg/kg | interferes with establishment of conditioned nausea-like behavior and attenuates the established conditioned nausea-like behavior | 89 | ||
| Alzheimer disease (AD) | suppression of neuroinflammation | Animal models of AD | 2.5-100 mg/kg | memory improvement, prevention of cognitive deficits, ↓ Aβ plaques | 90 |
PPI: prepulse inhibition; EPM: elevated plus maze tests; FST: forced swim test; AD: Alzheimer's disease.
Among the few therapeutic actions explored in well-controlled human trials are those related with CBD effects on psychosis, Parkinson's disease (PD), epilepsy, and anxiety (Table 6). The main findings of these trials are as follows:
Epilepsy
The strongest evidence supporting CBD medical use is the one associated with the treatment of various types of epilepsy. Preclinical studies showed a decrease in the proportion of animals exhibiting seizures and reduced seizure-related mortality in several animal models of epilepsy (Table 7). Epidiolex®, the FDA-approved CBD preparation, has been reported to importantly decrease the median number of monthly seizure episodes in a number of clinical trials that included patients with severe, treatment-resistant, and childhood-onset seizures; Lennox-Gastaut patients, Dravet syndrome patients, patients with tuberous sclerosis complex, and adults and children with other types of epilepsy (reviewed in Britch et al.60) (Table 6).
Psychosis
CBD has been postulated as a potential treatment for psychosis (for review, see75,76). The antipsychotic potentiality of CBD was suggested by the detection of increased eCB circulating levels and changes in cannabinoid receptor expression in schizophrenic patients77, as well as by preclinical evidence showing CBD-induced reduction of distinct psychotic-like symptoms in animal models (Table 7). At this moment, two trials with schizophrenic patients showing a reduction in psychotic symptoms and one trial with patients with PD, a neuropsychiatric disease that may include psychotic symptoms, have been published. In this last case, the data of one clinical trial indicate that CBD reduced the psychotic symptoms related to PD and, in another, that it improved overall PD symptoms60 (Table 6).
Likewise, the psychotic outcomes associated with Δ9-THC exposure91 can be prevented or reversed by CBD both in humans92 and in animal models93. Moreover, the habitual use of Cannabis preparations with relatively high CBD concentrations produces fewer psychotic experiences than those with lower CBD content94. Notwithstanding, a recent controlled study found no evidence of CBD modulating Δ9-THC-elicited effects95.
Anxiety
Preclinical studies have reported CBD-mediated prevention of stress-induced anxiety-like responses in several animal models and reduction of already-induced anxiety-like behaviors (Table 7). Clinical investigations on the potential anxiolytic properties of CBD have been conducted in a couple of trials with healthy adults and two other studies were run in patients with anxiety disorders. The randomized, double-blind, and placebo-controlled studies mainly report on CBD-induced decreases in social anxiety in these two populations and in a trial with patients with fragile X syndrome, in which CBD produced what the authors called "meaningful clinical reduction" of social avoidance and of the anxiety component of this disease. Interestingly, trials with healthy adults found anxiolytic effects at a specific CBD oral dose (300 mg), while lower and higher CBD doses lacked effects on anxiety in this population64. Other studies employing CBD doses above 300 mg did not find anxiolytic effects in healthy subjects96. In contrast, studies in patients with anxiety disorders found anxiolytic effects at higher CBD doses (400 or 600 mg)64 but also at lower doses (100-250 mg)74 (Table 6). These data suggest that CBD might exert biphasic, dose-related effects on anxiety and that the basal anxiety level might modify the "therapeutic dose window." Notwithstanding, the evidence provided by the mentioned studies for the CBD management of anxiety disorders is weak since it is mainly centered on a specific type of anxiety, social anxiety. Therefore, high-quality studies supporting CBD's effects on other anxiety expressions are required.
Other potential therapeutic actions of cannabidiol
Other therapeutic applications endorsed to CBD include the relief of depressive symptoms, its usefulness as an antiemetic agent, and its ability to provide neuroprotective effects in Alzheimer's disease due to its antioxidant and anti-inflammatory properties97. However, the support for these effects relies only on preclinical studies (Table 7). Reduction of depressive-like behaviors and increases in motivation and hedonic behaviors in animal models of depression has been reported to result from CBD acute and chronic treatments. To date, there are no clinical trials that replicate these CBD properties.
CBD's reduction of nausea and vomiting has also been shown in animal models of conditioned and unconditioned nausea-like behavior. However, again, there are no clinical trials aimed at confirming this property in humans.
Due to its antioxidant and anti-inflammatory properties, CBD has been found to exert neuroprotective effects in animal models of Alzheimer's disease (AD), although no clinical trial has yet been conducted.
Overall, the potential therapeutic uses of cannabinoids are promising; however, the pharmacological manipulation of a neuromodulatory system like the ECS is challenging because the consequences of such manipulations may affect different neural functions that are difficult to foresee. An example is the experience with Rimonabant, a CB1R antagonist that was marketed in Europe from 2006 to 2009 as a treatment for overweight and type II diabetes management that had to be retired due to severe psychiatric side-effects, including depression and suicidal ideation, not detected during the clinical trials68.
CONCLUSIONS
The evidence presented in this review allows us to conclude that Δ9-THC and CBD produce biphasic, dose-dependent effects on several physiological responses. The biphasic nature of cannabinoid effects highlights the need for a careful analysis of the dose ranges separating therapeutic from unwanted effects. Besides, Δ9-THC produces dependence and harmful effects, while CBD does not.
The available pharmaceutical formulations of these phytocannabinoids and related molecules have clear therapeutic effects; however, more research is needed to assure their efficacy and safety since their effects are complex. Several additional potential therapeutic applications, mainly for CBD, are being proposed which, however, still lack sufficient clinical and preclinical support. For this reason, caution is advised when using or prescribing cannabinoids.
Additional consideration deserves the fact that the ECS participates in the development of the central nervous system98. Therefore, the use of cannabinoid-based formulations for the medical treatment of children and adolescents experiencing brain developmental changes must be carefully examined, balancing the pros and cons of prescribing cannabinoid-based medications in every single case. Cannabis research field is expanding with the identification of new molecular targets, the characterization of undescribed phytocannabinoids, and novel findings related to the ECS99. In addition, consumption of synthetic cannabinoids as drugs of abuse represents a new challenge in addiction research100.











 nueva página del texto (beta)
nueva página del texto (beta)


