Introduction
Cancer is a multifactorial disease and the second cause of death of general population in the world. Its causality is related to lifestyle, genetic predisposition and psychological and mood states of individuals.1 The prevalence of cancer has increased in recent years, and it is therefore a health problem that demands a very high level of attention.2,3 In males, the highest rates of cancer incidence occur in the prostate, lung, bronchi, colon, rectum and urinary bladder; in females, in the breast, lung, bronchi, colon, rectum, uterus and thyroid gland. The above is an indicator that the most common types of cancer in men and women, respectively, are prostate and breast cancer.4 In children, the highest incidence of cancer is observed in the bone marrow, brain and lymph nodes.5,6
Epithelial cancer is one of the most aggressive types. Particularly in prostate cancer, the treatment will depend on the stage the disease is identified at; however, it is very common for it to progress and develop a phenotype of apoptosis resistance and, therefore, of hormone independence, which makes for cells to continue proliferating until metastasis is reached, hence the development of new therapeutic strategies that prevent the disease from reaching advanced stages being essential.
During the process of apoptosis of numerous cell types, a sustained increase in cytoplasmic Ca2+ has been defined as a determinant in the onset of programmed cell death.7 Considering this rationale, for years we have worked on the study of the mechanisms of cytoplasmic calcium homeostasis control and the impact of Ca2+ altered level in various cell and neoplastic types.
We have been able to demonstrate that an increased level of cytoplasmic calcium results in the onset of programmed cell death in Chinese hamster epithelial cell lines of prostate and ovarian cancer. In this sense, our research group discovered a nonselective cationic channel permeable to Ca2+ with 23pS-conductance, expressed under conditions of induced apoptosis by removing serum from the culture medium in androgen-independent lymph node carcinoma of the prostate (LNCaP) cells.8 In addition, it was also possible to isolate the complementary DNA (cDNA) from LNCaP cells that encodes apoptosis regulatory protein 2 (which we called ARP2), which, when overexpressed, induces apoptosis in Xenopus laevis oocytes and androgen-independent LNCaP cells, from which ARP2 was originally isolated.9 ARP2 overexpression was shown to induce Chinese hamster ovary cell apoptosis. These findings demonstrate that Ca2+ increased levels are an indispensable condition to initiate apoptosis in various epithelial cell lines, and this protein could therefore constitute an excellent tool in the future in the treatment of the disease by preventing progression to the advanced stages that culminate with a metastatic process.
Prostate cancer
Prostate cancer is the second leading cause of cancer-related death in the United States and the fifth in the world.10-12 The prevalence of this disease increases with increasing age. Prostate cancer is found during autopsy in more than half of men older than 50 years in the United States, in spite of this disease beimng the cause of death only in 3 %. It is an asymptomatic disease until the appearance of metastatic lesions that are usually discovered in bone tissue. Initial therapies for its treatment include surgery, radiation, and use of 5-alpha reductase inhibitors, which promotes the formation of more potent androgens from testosterone.13 Initial treatment methods often cause sexual, urinary and intestinal dysfunction.14
From the molecular point of view, this disease manifests itself at early and late stages. In the former, prostate cell proliferation is slow and dependent on androgens, so that during treatment with chemotherapeutic agents, the cells manage to repair the damage and continue to proliferate. Over time, cells become independent of androgenic hormones to proliferate, which culminates in the development of metastases, leading to patient death.15
Apoptosis and disease
Programmed cell death or apoptosis is an intrinsic cellular event of relevant importance in processes such as cell homeostasis, embryonic development and onset and maintenance of several diseases such as cancer and atherosclerosis.16,17 This mechanism develops through two pathways; the cell death receptor and the mitochondrial pathway.18 In turn, the apoptotic process develops in several stages: the first one is related to the stimuli that trigger programmed cell death, the second involves the signal transduction processes, in the third, effector enzymes that take care of cell disassembly, such as active caspases, participate and, finally, in the fourth, chromatin condensation, DNA degradation and apoptotic body formation occurs.18,19
There are various apoptotic pathways, such as the extrinsic or death receptor pathway, whose initiating caspase is caspase 8, and the intrinsic or mitochondrial pathway, which has cytochrome C as intermediary protein and caspase 9 as initiator. Both can converge in the set of effector caspases, mainly caspases 3 and 7, which are activated through self-processing or cascade activation, whereby caspases themselves are self-activated and activated between each other.20,21 There is another alternative pathway called the perforin-granzyme pathway,22,23 which corresponds to an important serine protease complex in apoptosis that is induced by cytotoxic T cells by activating independent caspase pathways.
Ca2+ as the second determining messenger in the apoptotic process
Variations in intracellular Ca2+ concentration promote the onset of cellular events such as the regulation of metabolism, mitosis, secretion of neurotransmitters and hormones, as well as the contraction of myofilaments, and therefore it is considered a second messenger that is determinant for cell functions.24,25 Ca2+ levels are also involved in the regulatory mechanisms of apoptotic programmed cell death.26,27 A Ca2+ level higher than basal is considered highly toxic, since it generates the activation of proteases and phospholipases that participate in cell disassembly.28 The increase in Ca2+ can occur at early and late stages of apoptosis, through the outflow of Ca2+ from the endoplasmic reticulum and Ca2+ inflow to the cytoplasm through channels activated by Ca2+ release.7,29 It is necessary taking into account that part of intracellular Ca2+ moves to the endoplasmic reticulum through the endoplasmic reticulum calcium pump and that Ca2+ is released from these stores by inositol 1,4,5 triphosphate receptors or ryanodine receptors. Additionally, in various intracellular organelles (such as the Golgi apparatus, nucleus and mitochondria) there are specialized systems for Ca2+ transport.30
Calcium enters the cell through transmembrane proteins called calcium channels.31 Calcium passes through the channels by means of different mechanisms, depending on the type of channel or voltage, or through receptors; these pathways do not require energy, unlike calcium pumps, which release cytoplasmic calcium to the exterior of the cell at the expense of the use of adenosine triphosphate.
In collaboration with Dr. Agustín Guerrero of CINVESTAV, in the laboratory of the Institute of Cellular Physiology, National Autonomous University of Mexico, for the obtainment of electrophysiological records, the membrane fixation technique (patch clamp) and single cell simultaneous Ca2+ measurements were combined, with the purpose of studying the activation of Ca2+-permeable channels using two different inducers: an ionophore (ionomycin) and serum elimination.8 The latter deprives the cells from essential nutritional components such as proteins, growth factors and vitamins, inducing cells to death.32,33
The results demonstrated the activation of a non-selective and Ca2+-permeable cationic channel with 23pS-conductance. Ca2+ increased levels induced cells to apoptosis, which demonstrated that activation of this channel promotes the development of LN-CaP cells programmed death, which in turn constitutes an important finding for programmed death induction in this cell type (Fig. 1).
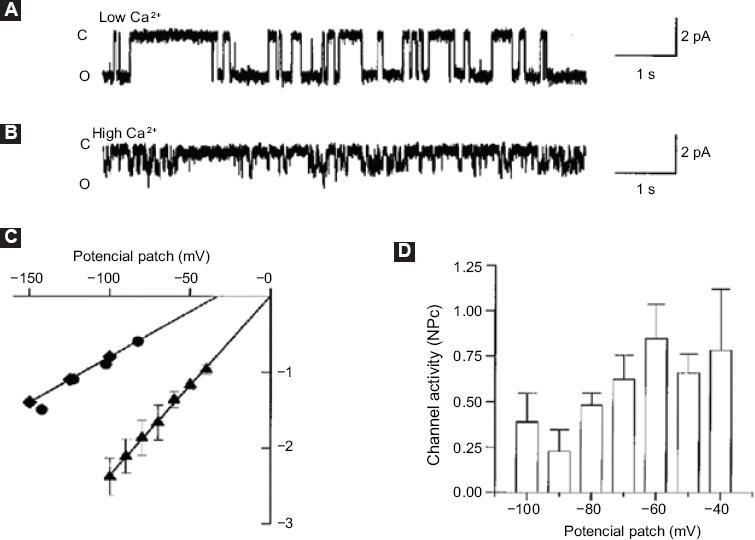
Figure 1 Characterization of ion channels associated with the second Ca2+increase. A: Simple channel current records in the adhered cell configuration with a potential patch of -100 mV and Na+-Ca2+ solution; B: Simple channel records with 110 mM CaCl2 in the pipette solution and a potential patch of -100 mV. The closed (C) and open (O) levels are indicated on the left side of the record; C: Current-voltage curves obtained in Na+Ca2+ (▴) solution, 110 mM CaCl2 (♦) or 110 mM calcium glutamate () as charge carriers; D: In the indicated membrane potentials (n = 4), mean ± standard deviation of basal state activity of the 23pS channel is shown. Taken from reference 8.
Continuing with this line of research, in our group, cDNA arp 2, which codes for ARP2 of androgen-independent LNCaP cells, and which were induced to apoptosis by serum removal, was isolated, identified and characterized.9 The sequence of this protein shows homology with the splicing factor prp8 (a component of the spliceosome)34 and proapoptotic functions in different cell types (Fig. 2). The alternative-type splicing mechanism is defined as a property that prevails in higher organisms to produce multiple proteins from a simple gene.35,36 Prp8 protein ubiquitin-binding activity suggests that some splicing factors for pre-mRNA can be ubiquitinated to have an interaction with Prp8.37
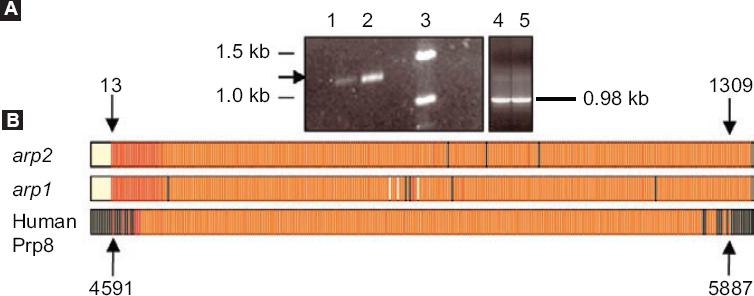
Figure 2 arp1 and arp2 cloning A: 1 % (w/v) agarose, arp2 polymerase chain reaction products. Lane 1, product of cells growing in the presence of serum (control). Lane 2, product of cell cultures in the absence of serum. Lane 3, molecular weight markers (GIBCO BRL, DNA 1 kb). Lanes 4 and 5, GAPDH controls; B: arp1 and arp2 cDNA multiple alignment with human Prp8 cDNA. Nucleotides 4591-5887 correspond to the human Prp8 region that overlaps with arp1 and arp2 cDNA sequences. The black lines indicate the nucleotides that do not show homology with cDNAs. The yellow spaces between the red lines correspond to nucleotide empty spaces between cDNAs. The black arrows point at arp1 and arp2 nucleotides 13 and 1309 and Prp8 nucleotides 4591 and 5887. Taken from reference 9.
Xenopus laevis frog oocytes injected with arp2 mRNA showed blistering after 12 hours of injection; these morphological changes were observed to increase when the oocytes are treated with thapsigargin (Fig. 3). It was also possible to observe that the oocytes suffered a decrease in the resting membrane potential: from a control value of -46.8 ± 6.6 mV they went to -5.9 ± 3.4 mV (5-8 oocytes, two frogs) (Fig. 4). Incubation of the cells with thapsigargin accelerated and increased morphological changes, with the loss of definition of the animal and plant poles being evident, which was observed 36 hours after injection with arp2 mRNA and eight hours after incubation with thapsigargin (Figure 3B).
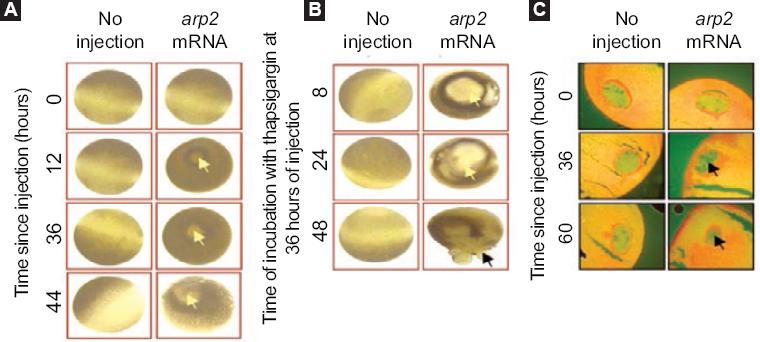
Figure 3 Xenopus laevis oocytes morphological apoptosis-related changes after arp2 mRNA injection; A: Apoptosis induction in Xenopus laevis oocytes by arp2 mRNA microinjection. Vertical columns correspond to oocytes that did not receive injection and oocytes injected with arp2 mRNA. Horizontal lines show injection times (0, 12, 36, 44 hours). The yellow arrows show the blisters formed; B: Cell death progression in Xenopus laevis oocytes injected with arp2 mRNA and incubation with 5 µM of thapsigargin. Vertical columns show control oocytes that did not receive injection and oocytes injected with mRNA. Horizontal lines show incubation times with thapsigargin at 36 hours of injection and 8, 24, and 48 hours of incubation with thapsigargin; C: Morphological changes in the nucleus of Xenopus laevis oocytes observed after arp2 mRNA injection. Vertical columns show histological sections of non-injected oocytes and oocytes injected with arp2 mRNA. Horizontal lines show incubation time after mRNA injection (0, 36, 60 hours). The black arrows show chromatin condensation. Taken from reference 9.
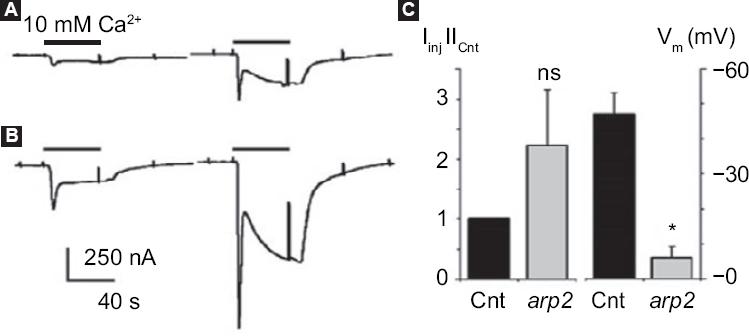
Figure 4 arp2 mRNA functional expression in Xenopus laevis oocytes. Ion currents generated by two consecutive pulses of 10 mM Ca2+ in Ringer-Mg2+ solution after oocyte incubation in the absence of Ca2+ and in the presence of 5 µM of thapsigargin; A: Incoming currents generated in a non-injected control oocyte; B: Current in an oocyte injected with mRNA arp2. Histogram showing the mean ± standard deviation of the current generated in control oocytes (n = 3) and oocytes injected with arp2 mRNA (n = 6). Changes in resting potential (Vm) observed between the different groups of oocytes (eight controls and six injected) are also shown. All oocytes of this figure were from the same donor; similar results were obtained from a second frog. Cnt = control, ns = not significant, * p = 0.05. Taken from reference 9.
The morphological changes and membrane depolarization observed in our study have been described in Xenopus laevis oocytes when injected with cytochrome C to trigger the apoptotic mechanism,38 as well as in oocytes injected with the Bcl-xs proapoptotic molecule.9,39 Thapsigargin, a drug that depletes Ca2+ intracellular stores by the specific inhibition of endoplasmic reticulum Ca2+-ATPase, elicits the activation of cytoplasmic membrane-independent voltage channels (TRP, transient receptor potential).40
This way, we have gathered sufficient evidence that ARP2 is an apoptosis promoter in androgen-independent LNCaP cells and in Chinese hamster ovary epithelial cells,41 thus favoring Ca2+ sustained increases by directly interacting with Ca2+-permeable membrane channels or by presenting a membrane channel function. On the other hand, considering the homology of the cDNA sequence that encodes ARP2 with the Prp8 splicing factor, it is not yet known whether this protein could additionally have any participation in the assembly or functioning of the spliceosome or be related to alternative splicing regulation mechanisms of mRNAs that encode proteins of the apoptotic machinery. There is a study where the Prp8 protein was observed to bind to one of the androgen receptor domains of prostate cancer cells and that, consequently, could be intervening in its functionality during the development of the disease.42 Furthermore, some splicing factors that make up the spliceosome, such as the SNW1 factor, have been reported to be linked to the development of breast cancer.43
Continuing with the project, ARP2-encoding cDNA was cloned in an expression plasmid and transfected to androgen-independent LNCaP cells and Chinese hamster ovary cells. ARP2 overexpression induced the cells to develop apoptosis, with an important impact on cell viability and effector caspases 3 and 7 activation, which are comparable results to those of starving cells treated with ionomycin41 (Fig. 5). In this study, using confocal microscopy, it was possible to demonstrate that ARP2 is initially located in the perinuclear region of cells and migrates over time to the plasma membrane41 (Fig. 5). Taking into account our results, we consider that ARP2 is inserted into the plasma membrane, with a function similar to that of a membrane channel, thus constituting a valuable target to modulate Ca2+ flow and concentration in the cytoplasm of epithelial cancer cells that show an apoptosis-resistant phenotype41 (Fig. 6).
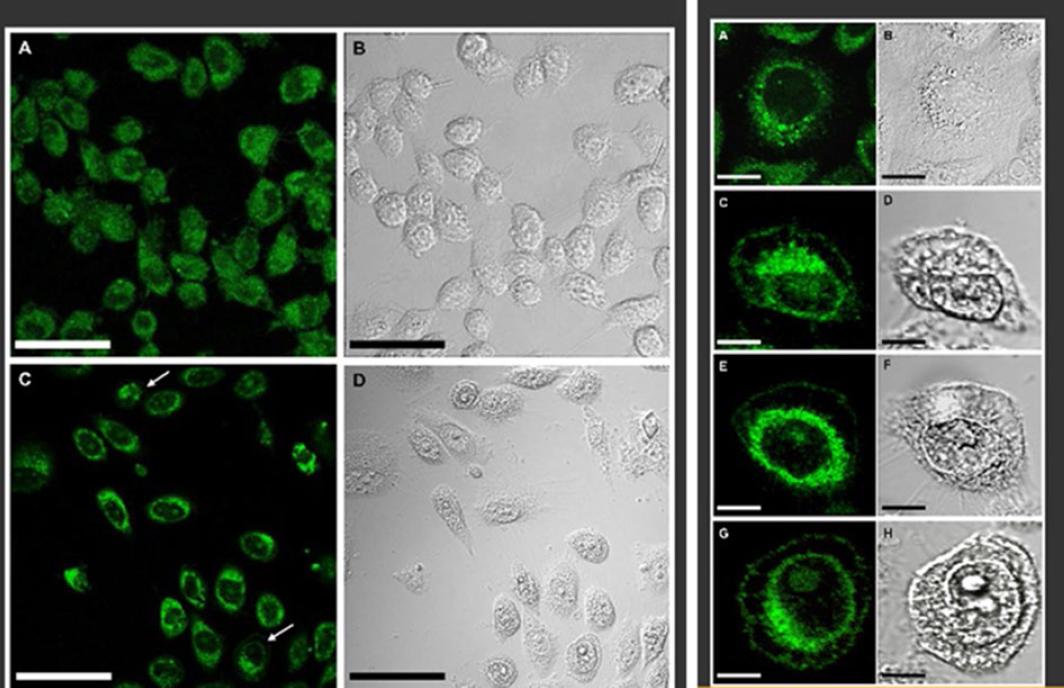
Figure 5 Confocal microscopy of the ARP2 fusion protein expressed in Chinese hamster ovary (CHO) cells. CHO cells transfected with egfp cDNA were observed at 24 hours post-transfection. A: Differential interference contrast microscopy; B: CHO cells transfected with arp2-egfp cDNA were also observed 24 hours post-transfection; C: Differential interference contrast microscopy; D: Bar scale: 50 µm. White arrows indicate that ARP2 is localized in the perinuclear region. Right side: CHO cells transfected with arp2-egfp cDNA examined at 16, 24, 48 and 72 hours post-transfection (A, C, E and G, respectively); same as above, but with differential interference contrast microscopy (B, D, F and H, respectively). Bar scale: 10 µm. Taken from reference 41.
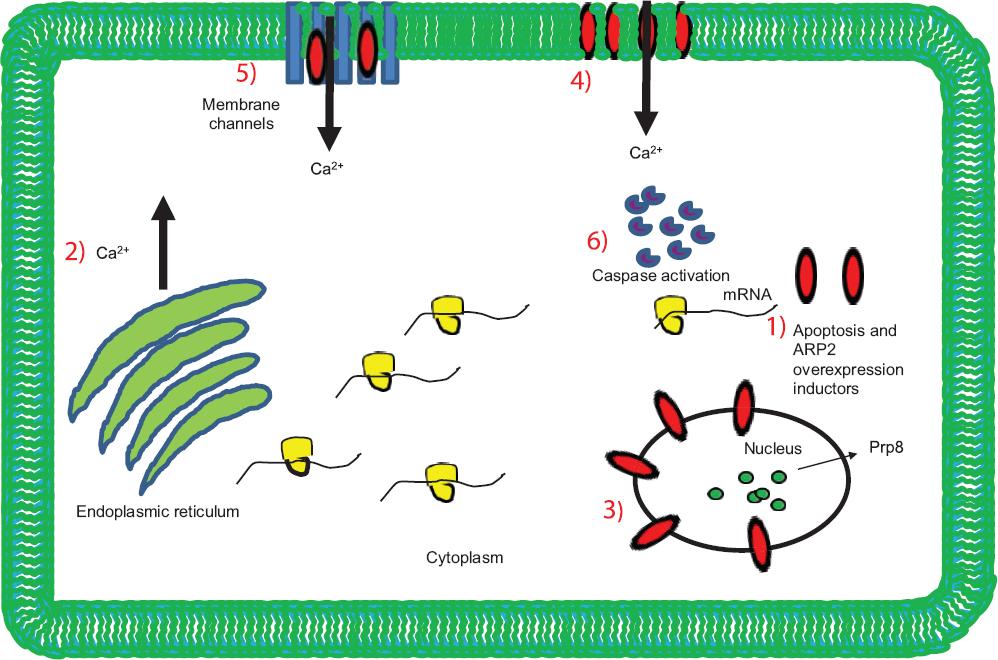
Figure 6 ARP2 action model. 1: Inducers of ARP2 apoptosis and overexpression; 2: ARP2 overexpression promotes an increase in Ca2+ that originates in the endoplasmic reticulum and cytoplasmic membrane; 3: ARP2 localized in the perinuclear membrane in interaction with the spliceosome complex, which promotes Ca2+ inflow; 4: ARP2 inserting into the cytoplasmic membrane and thereby forming possible membrane channels or regulating the activation of Ca2+-permeable channels (5): these Ca2+ inflows favor spliceosome complex activity and the development of the apoptosis mechanism (6).
Perspectives
The apoptosis mechanism in malignant cell resistant phenotypes has been the subject of extensive study in recent years. Due to the strong impact of cancer development, implementation of successful molecular strategies that support treatments against this disease is urgent. Our group has demonstrated that ARP2 protein overexpression induces programmed cell death in different cell types: androgen-independent lymphoid nodule prostate cancer cells, from where it was originally isolated, Chinese hamster ovary cells, embryonic human kidney cells and coronary artery endothelial cells (data not shown).
Given the collected experimental evidence, we consider that ARP2 could be contributing during its overexpression to the increase in Ca2+ intracellular levels as a messenger molecule or as a protein inserted in the membrane that favors Ca2+ inflow. In addition to being found in the membrane region, we also observed its perinuclear localization, and due to the homology with the Prp8 factor, there is the possibility that it participates in the alternative splicing mechanisms of pro-apoptotic molecules.44-46 In this sense, and taking into account that transcription through alternative splicing mechanisms are molecular processes that are highly regulated by Ca2+ intracellular level, among other factors,47,48 it is feasible to think that ARP2 might have a double function: in the control of plasma Ca2+ flow and in alternative splicing molecular mechanisms.
Based on the evidence that cell death genes are deregulated in cancer, a phenomenon that is associated with the control of their alternative splicing patterns, it is reasonable thinking that the apoptosis process occurs as a positive response to chemotherapy.49 If we take into account that anticancer drugs efficacy may also depend on apoptosis activation or on a form of cell-senescence acute induction, we consider that ARP2 protein overexpression could potentially be used as a new tool in the treatment epithelial-type cancer.











 nueva página del texto (beta)
nueva página del texto (beta)


