Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de fitopatología
versión On-line ISSN 2007-8080versión impresa ISSN 0185-3309
Rev. mex. fitopatol vol.38 no.3 Texcoco sep. 2020 Epub 27-Nov-2020
https://doi.org/10.18781/r.mex.fit.2004-5
Review Articles
The Burkholderia genus: between mutualism and pathogenicity
1 Laboratorio Interacción Molecular Planta-Microorganismo, Programa de Edafología Colegio de Postgraduados, Carretera México-Texcoco Km 36.5, Montecillo Estado de México, México, 56230;
2 Laboratorio Microbiología de Suelos, Benemérita Universidad Autónoma de Puebla, Avenida San Claudio s/n, Ciudad Universitaria, La Hacienda, Puebla, Puebla, México, 72592;
Burkholderia is an ambivalent genus because some of its species establish symbiotic-mutualistic relationships with plants, and symbiotic-pathogenic relationships with plants, animals, and humans. Since the phytopathogenic bacterium B. cepacia was reported as a nosocomial opportunist, associated with cystic fibrosis, the concern about possible infections in humans arose. The objective of this contribution was to make an analysis of Burkholderia’s functional versatility and its effect on human health. Burkholderia harbored about 100 species and the B. cepacia complex (BCC) consisting of 22 species. At the beginning, the existence of two lineages within the genus was determined: the A that included several species that were associated with plants, as well as the saprophytes; and B containing BCC species (human pathogenic opportunists), the B. pseudomallei subgroup that included human and animal pathogens, and a group of plant pathogenic species. Finally, some individuals were renamed as Paraburkholderia and Caballeronia. Recent analyzes of burkholderias from humans and the environment indicate that there is no phylogenetic subdivision that distinguishes between beneficial and pathogenic ones. Hence the importance of considering risks to human health, when any member of this group is employed in agricultural activities.
Key words: Burkholderia; Paraburkholderia; Caballeronia; mutualism; parasitism; cystic fibrosis
Burkholderia es un género ambivalente debido a que algunas de sus especies establecen relaciones simbiótico-mutualistas con las plantas, y simbiótico-patogénicas con plantas, animales y humanos. Desde que la bacteria fitopatógena B. cepacia fue reportada como oportunista nosocomial, asociada a la fibrosis quística, surgió la preocupación de posibles infecciones en humanos. El objetivo de esta contribución fue hacer un análisis de la versatilidad funcional de Burkholderia y de su efecto en la salud humana. Burkholderia albergó cerca de 100 especies y al complejo B. cepacia (CBC) constituido por 22 especies. Inicialmente, se determinó la existencia de dos linajes dentro del género: el A que incluía varias especies que se asociaban con plantas, así como las saprofitas; y el B que contenía las especies del CBC (oportunistas patógenas de humanos), el subgrupo de B. pseudomallei que incluía patógenos de humanos y animales, y un grupo de especies fitopatógenas. Finalmente, se renombraron algunos individuos como Paraburkholderia y Caballeronia. Los análisis recientes de burkholderias provenientes de humanos y del ambiente, indican que no existe una subdivisión filogenética que distinga entre benéficas y patogénicas. De ahí la importancia de considerar los riesgos para la salud humana, cuando algún miembro de este grupo sea empleado en actividades agrícolas.
Palabras clave: Burkholderia; Paraburkholderia; Caballeronia; mutualismo; parasitismo; fibrosis quística
The Burkholderia genus includes non-spore-forming Gram-negative bacilli, which, depending on the species, uses one or several polar flagella to move. Individuals that belong to the Burkholderia genus have a G + C ratio between 59 and 69.5%, are aerobic, oxidase and catalase positive, mesophilic, and synthetize polyhydroxybutyrate as reserve material (Figure 1). The members of the Burkholderia group are ubiquitous since they are found in water and soil, as well as in plant and animal tissues (Garrity et al., 2006). Nutritional versatility is one outstanding characteristic of this bacterial group because it can live as a biotroph or as a saprophyte.
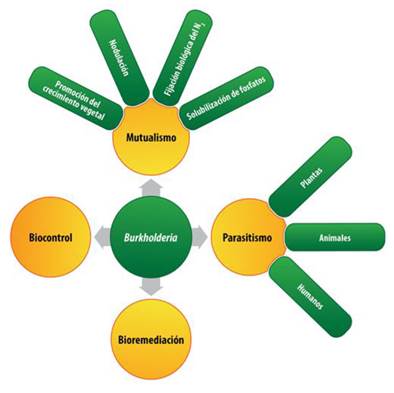
Burkholderia is a bifunctional genus because some of its species establish symbiotic-mutualistic relationships with plants, while others establish symbiotic-pathogenic associations with plants, animals, and humans. Figure 2 shows the functional versatility of the Burkholderia genus.
Classification of the Burkholderia genus. William Burkholder described Pseudomonas cepacia for the first time as the causal agent of onion rot (Burkholder, 1950). Subsequent isolations indicated that P. cepacia was a versatile group formed by isolates that can establish mutualistic and pathogenic interactions. Due to the phenotypic diversity observed in P. cepacia isolates, it was reclassified within the pseudomonadals order.
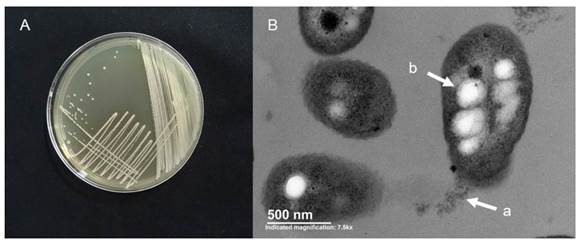
Figure 1. A, Burkholderia cepacia BUAP-AM51 culture (accession KX161745) growing in trypticasein-soybean agar. B, Microphotography of the same strain, using transmission electron microscopy, where the polar flagellum (a) and polyhydroxybutyrate granules (b) are observed.
The Burkholderia genus was proposed by Yabuuchi et al. (1992) as a result of the transference of seven Pseudomonas species: P. cepacia, P. mallei, P. pseudomallei, P. plantarii, P. caryophylii, P. pickettii and P. solanacearum. Later, Burkholderia pickettii and B. solanacearum were transferred to the Ralstonia genus (Yabuuchi et al., 1995). The Burkholderia genus includes around 100 species (Estrada-de los Santos et al., 2015) distributed in diverse ecological niches. Particularly, BCC is ubiquitous in nature and can be found in soil, water, rhizosphere, several animal species, and hospitals (Chiarini et al., 2006). Currently, BCC is formed by 22 species (Table 1).
All the BCC species exhibit considerable phenotypic variability (Vandamme et al., 1997), even within clinical isolates of the same species (Larsen et al., 1993). Probably, the BCC ecological versatility is caused by the unusual size of its genome, which normally consists of two to four (typically three) large replicons (chromosomes) (>500 kb), as well as by its ability to use a long list of compounds as carbon sources. However, its taxonomic classification does not permit to clearly distinguish the strains that are pathogenic to humans (Parke and Gurian-Sherman, 2001).
Table 1. Species of the Burkholderia cepacia complex (BCC)
| Núm. | Especie | Referencia |
|---|---|---|
| 1 | B. alpina | Rojas-Rojas et al., 2018 |
| 2 | B. ambifaria | Coenye et al., 2001 |
| 3 | B. anthina | Vandamme et al., 2002 |
| 4 | B. arboris | Rojas-Rojas et al., 2018 |
| 5 | B. cenocepacia | Vandamme et al., 2003 |
| 6 | B. cepacia | Vandamme et al., 1997 |
| 7 | B. contaminans | Rojas-Rojas et al., 2018 |
| 8 | B. diffusa | Rojas-Rojas et al., 2018 |
| 9 | B. dolosa | Vermis et al., 2004 |
| 10 | B. lata | Rojas-Rojas et al., 2018 |
| 11 | B. latens | Rojas-Rojas et al., 2018 |
| 12 | B. metallica | Rojas-Rojas et al., 2018 |
| 13 | B. multivorans | Vandamme et al., 1997 |
| 14 | B. paludis | Rojas-Rojas et al., 2018 |
| 15 | B. pseudomultivorans | Rojas-Rojas et al., 2018 |
| 16 | B. pyrrocinia | Vandamme et al., 2002 |
| 17 | B. seminalis | Rojas-Rojas et al., 2018 |
| 18 | B. stabilis | Vandamme et al., 2000 |
| 19 | B. stagnalis | Rojas-Rojas et al., 2018 |
| 20 | B. territorio | Rojas-Rojas et al., 2018 |
| 21 | B. vietnamiensis | Gillis et al., 1995 |
| 22 | B. ubonensis | Vermis, et al., 2002 |
As the number of described Burkholderia species increased, the analysis of multilocus sequences (atpD, gltB, lepA, and recA) and 16SrRNA sequencing revealed that the genus was formed by at least two lineages, A and B (Estrada-de los Santos et al., 2013). Lineage A was originally divided in two sub-lineages: saprophyte species and species associated with plants, including those that induce the formation of nodules in legumes (Angus et al., 2014). Lineage B was formed by BBC members that are opportunistic pathogens in human beings; animal and human pathogens of the B. pseudomallei group; phytopathogenic species; and some saprophyte species. The B. andropogonis and B. rhizoxinica/B. Endofungorum species not included in any of the two lineages could probably be new genera (Martínez-Aguilar et al., 2013). The existence of intermediate groups between lineages A and B questioned if this evidence was enough to separate the Burkholderia genus (Estrada-de los Santos et al., 2015).
Forty-five Burkholderia species of lineage B were separated using specific conserved sequence indels (CSIs) as molecular markers. Based on the results, the description of the Burkholderia genus of lineage B was amended. The CSIs markers are not found in the genome of environmental burkholderias of lineage A. Nevertheless, environmental bacteria of lineage A, along with the phytopathogenic species B. andropogonis, B. rhizoxinica and B. endofungorum were transferred to the new Paraburkholderia genus with Paraburkholderia graminis as a type species (Sawana et al., 2014).
During the period when about 46 species of the Paraburkholderia genus were validated and published in the International Journal of Systematic and Evolutionary Microbiology (Oren and Garrity, 2015), 16 new species of the Burkholderia genus were also described. Of these species, B. stagnalis and B. territorii were very similar remarkably similar to the 16S rRNA gene sequence of B. glumae, and very similar to the sequence of 7 fragments of B. ubonensis and B. latens housekeeping genes (De Smet et al., 2015). However, given the similarities of the remaining 14 species with species of the Paraburkholderia genus, the reclassification of the species of the Burkholderia genus, proposed by Sawana et al. (2014), was considered. Thus, 11 species of the Burkholderia genus were transferred to the Paraburkholderia genus, and the new Caballeronia genus was proposed, to which the remaining three Burkholderia species were transferred (Dobritsa and Samadpour, 2016).
New Burkholderia species have been described. In a study in which 17 isolates from human beings and the environment were analyzed through a partial sequencing of the gyrB gene, which is used in multilocus sequencing to identify burkholderias that have not yet been classified, 13 bacterial genomovars were determined and identified as Burkholderia glathei Clade bacteria (BGC). These isolates represented 13 new Burkholderia species, which were distinguished by their genotypic and phenotypic traits as: B. arvi, B. hypogeia, B. ptereochthonis, B. glebae, B. pedi, B. arationis, B. fortuita, B. temeraria, B. calidae, B. concitans, B. turbans, B. catudaia and B. peredens (Peeters et al., 2016).
Volcanic soils are a source of isolation of new CO-oxidizing species. Weber and King (2017) described Burkholderia alpina, from the Pico de Orizaba volcano, and Paraburkholderia hiiakae, P. paradisi, P. peleae and P. metrosideri from the Kilauea volcano. All the isolates had the coxL gene that encodes the catalytic subunit of carbon monoxide dehydrogenase.
Recently, Jin et al. (2020) reported 36 genomovars within the BCC when they used the whole genome of 116 Burkholderia strains. Based on these results, the authors suggested a new BCC re-arrangement because 22 genomovars corresponded to the BCC species known so far, while the remaining 14 are probably new species that should be added to the BCC.
Burkholderia as a phytopathogenic agent. There are many anthropogenically important crops infested by different Burkholderia species. Since Burkholder’s report in 1950 up to the beginning of the 21 century, reports of plant diseases caused by this controversial bacterial genus have increased (Table 2).
The presence of members of the B. cepacia complex is common in agricultural soil. Jacobs et al. (2008) characterized 1,290 Burkholderia isolates in onion plots (980 from rhizosphere and 310 from soil), of which 160 corresponded to B. cepacia, 480 to B. cenocepacia, 623 to B. ambifaria, and 27 to B. pyrrocinia. Most of the isolates of B. cepacia (85%), B. cenocepacia (90%) and B. ambifaria (76%) were pathogenic when inoculated in onion bulbs. Since B. gladioli was recently isolated from some orchid species (Table 2), this species can be considered as an emerging pathogen.
Table 5. Some Burkholderia pathogenic species of anthropogenically important crops.
| Especie | Hospedero/Enfermedad | Referencia |
|---|---|---|
| B. cepacia | Pudrición agria de la cebolla | Burkholder, 1950 |
| B. gladioli pv. alliicola | Piel resbaladiza de la cebolla | Burkholder, 1950 |
| B. glumae | Tizón bacteriano de la panoja del arroz | Shahjahan et al., 2000 |
| B. andopogonis | Mancha de la hoja del maíz | Vidaver y Carlson, 1978 |
| B. andopogonis | Raya bacteriana del sorgo, pasto sudan, teozintle y maíz dulce | Xin et al., 2009 |
| B. gladioli | Bacteriosis de los bulbos de Leucojum aestivum | Stoyanova et al., 2013 |
| B. tropica | Filodendro y helecho Boston | Ramírez-Rojas et al., 2016 |
| B. gladioli | Azafrán, maíz y arroz | Mirghasempour et al. 2018 |
| B. gladioli | Orquídeas Dendrobium sp., Oncidium sp. y Miltonia spp. | Keith y Thammakijjawat, 2019 |
Little is known about the virulence factors of the Burkholderia phytopathogenic species. One of the best documented cases is that of B. glumae, which causes rice bacterial blight (Quesada-González and García-Santamaría, 2014). When the environmental conditions are favorable, the bacterial density increases (quorum sensing) and induces the expression of its virulence factors such as synthesis of toxoflavin, flagella biogenesis, chemotactic response, type III secretion system and synthesis of the catalase enzyme (Kim et al., 2007; Quesada-González and García-Santamaría, 2014). The major damage factor is the synthesis of toxoflavin, a toxin that transports electrons between NADH and oxygen, without intermediation of cytochromes, and generates hydrogen peroxide, a highly toxic compound for plant tissue and microorganisms, which also obstructs the rice vascular bundles (Chung et al., 2009). The B. glumae strains that do not produce toxoflavin are avirulent and can be identified in the laboratory, because they do not produce the yellow pigment of toxoflavin in King B agar medium (Nandakumar et al., 2009). However, Suzuki et al. (2004) suggested that although the production of toxoflavin is a requirement for chlorosis in young panicles, it does not seem to play an important role in rot symptoms caused by B. glumae.
The pathogen is mainly transmitted by infected seed, on which it is transmitted to different regions. From seed germination until the seedling stage, rot is caused by a rapid increase of B. glumae populations in the plumules. Once B. glumae invades the spikelets, it multiplies rapidly and finally causes grain bacterial rot or panicle blight (Sayler et al., 2006).
The bacteria is dispersed by splashing but also by wind and rain, and by contact between panicles, but the disease occurs between 30-35 °C, especially at night, when there is frequent rainfall (Ham et al., 2011). B. gladioli also causes bacterial blight in the rice panicles. The symptoms of rice panicle blight and grain rot caused by B. gladioli are similar to those caused by B. glumae, that is, B. gladioli also produces toxoflavin (Nandakumar et al., 2009).
The bacterial blight of rice panicle has been a serious problem worldwide as it spread rapidly and caused significant rice production losses. The disease was reported for the first time in Japan in 1967, and then in Korea and Taiwan; in 2005 it was reported in the United States, and in 2006 and 2007 in Panama and Colombia, respectively (Pérez and Saavedra, 2011). B. glumae and B. gladioli infect the grain and produce similar symptoms, inducing 90 and 60% vain grain (empty hulls), respectively. Nonetheless, in rice fields that are severely affected by B. glumae, production losses of 75% have been reported because the bacterium causes additional damages such as seed germination inhibition, flower sterility and grain abortion (Nandakumar et al., 2009).
Burkholderia as plants mutualist symbiont. Interest in soil microorganisms has increased in recent years because they are essential to recycle nutrients, maintain soil fertility and biodegrade contaminating compounds. Among rhizobacteria, the group of plant growth promoting rhizobacteria (PGPR) is one of the most important because they establish symbiotic-mutualistic interactions with plant roots.
The biotroph members of the Burkholderia genus are mutually related to plants (Table 3) to which they nodulate and provide biologically fixed nitrogen, because of the presence of the nif genes (Caballero-Mellado et al., 2004). Similarly, they synthetize growth regulators, such as AIA, which promote plant growth (Angus et al., 2013). They also provide phosphorus for plant nutrition through a phosphate solubilization process (Gyaneshwar et al., 2002) and control disease causal agents (De los Santos-Villalobos et al., 2012).
The presence of B. vietnamiensis, B. cepacia and B. pseudomallei has also been detected on Gigaspora decipiens spores (Levy et al., 2003). The presence of nif genes in Burkholderia suggests that G. margarita obtains nitrogen from bacteria (Minerdi et al., 2001). New species of endosymbiotic burkholderias have been also described (Burkholderia rhizoxinica and B. endofungorum) in phytopathogenic fungi such as Rhizopus microsporus (Partida-Martinez et al., 2007).
Many endosymbionts of the Burkholderia genus are not cultivable. Thus, the endosymbionts of Psychotria kirkii leaf galls were classified as Candidatus Burkholderia kirkii (Van Oevelen et al., 2002). Similarly, the non-cultivable endosymbionts that live within arbuscular mycorrhizal fungi of the Gigasporaceae family (Bianciotto et al., 2000) were classified as Candidatus Glomeribacter gigasporarum (Bianciotto et al., 2003) due to its phylogenetic closeness to Burkholderia and to the possible presence of the nif genes (Minerdi et al., 2001). Finally, Van Borm et al. (2002) reported the presence of Burkholderia species in the organ in the form of a pouch in the middle part of the Tetraponera ant’s intestine. Those endosymbionts were phylogenetically related to B. fungorum and B. caledonica.
Table 3. Functions of Burkholderia species in a symbiotic-mutualistic relationship with anthropogenically important plants.
| Función | Burkholderia-Hospedero | Referencia |
|---|---|---|
| Promoción del crecimiento vegetal | B. cepacia - Zea mays | Singh et al., 2013 |
| B. cepacia - Cicer arietinum | Sánchez-Yáñez et al., 2014 | |
| B. ambifaria - Amaranthus cruentus | ||
| B. ambifaria - A. hypochondriacus | Parra-Cota et al., 2014 | |
| Nodulación | B. tuberum - Aspalathus carnosa | Vandamme et al., 2002 |
| B. phymatum - Machaerium lunatum | ||
| B. mimosarum, y B. nodosa - Mimosa bimucronata, M. scabrella y Dalbergia spp. | Chen et al., 2007 | |
| Fijación de N2 | B. vietnamiensis, B. unamae, B. tropica, | Caballero-Mellado et al., 2004 |
| B. xenovorans y B. kururiensis | ||
| Solubilización de P | B. tropica, B. unamae y B. cepacia -Lycpodium cernuum | Ghosh et al., 2016 |
Burkholderia and biological control. The Burkholderia genus also includes individuals that reduce or suppress the development of pathogens. Many soil-borne plant diseases caused by fungi and oomycetes are controlled by BCC individuals (Huang and Wong, 1998).
The most studied cases are related to the biocontrol of the damping-off or root rot disease (secadera) caused by Pythium spp. (Heungens and Parke, 2000), Rhizoctonia solani (Kang et al., 1998) and Fusarium spp. (Bevivino et al., 1998). The biocontrol of damping-off is essential because of the wide range of host plants and the fact that the seed is treated with fungicides that are deleterious to human health (Parke and Gurian-Sherman, 2001).
The damping-off disease can be effectively treated using biological controls given the short susceptibility period of the plant (hours or days), which will require protection for a short time. The specifically localized infection site allows a direct application of the biocontrol agent in the area where the plant requires protection. However, the biocontrol must be rapidly applied to prevent infection (Martin and Loper, 1999).
Among the antibiotics that are synthetized by the BCC members are cepacin A and cepacin B, which have antibacterial activity against staphylococci and toxicity in rats (Parker et al., 1984); cepaciamide A, which has fungicidal activity against Botrytis cinérea (Jiao et al., 1996); cepacidine A, which has antifungal activity against animal and plant pathogenic fungi such as Microsporum canis, Trichophyton spp, Epidermophyton spp, Fusarium oxysporum and Aspergillus niger (Lee et al., 1994); quinolinones, which promote Capsicum annuum growth and inhibit Phytophthora capsici growth, the causal agent of red pepper blight (Moon et al., 1996); phenylpyrroles, which inhibit the development of Fusarium sambucinum, the causal agent of potato dry rot (Burkhead et al., 1994); phenazine, which inhibits Rhizoctonia solani growth, the causal agent of poinsettia stem rot (Cartwright et al., 1995), and pyrrolnitrin, which contributes to suppress fungi such as R. solani and Fusarium (Parke and Gurian-Sherman, 2001).
On the other hand, the biocontrol of soil-borne pathogens is achieved using siderophores such as ornibactins, pyochelin and cepabactin that are synthetized in vitro by the BCC members (Sokol et al., 1999). In addition to the control of soil-borne pathogens, Knudsen and Spurr (1987) reported the effectiveness of some BCC individuals to control foliar fungal diseases.
Burkholderia and bioremediation. Microbial degradation is one of the most expedited routes to remove contaminants in terrestrial and aquatic environments. The Burkholderia sensu lato genus hosts individuals that are considered biodegraders or detoxifiers.
The B. vietnamiensis G4 strain of BCC is a species that efficiently degrades trichlorethylene (TCE), the most abundant organic contaminant in aquifers in the United States. The key in the TCE biodegradation process is the toluene o-monooxygenease enzyme (Tom), which is the first enzyme of the TOM operon encoded by the pTOM plasmid (Shields et al., 1995).
The B. xenovorans LB400 strain is one of the most effective aerial degrader of polychlorinated biphenyls (PACB), through the biphenyl-2,3-dioxygenase enzyme, which is encoded by the bhp locus (Ferrer et al., 2003).
The 2,4,5-trichlorophenoxyacetate (2,4,5-T), a potent herbicide component of the Orange Agent, is used by the B. phenoliruptrix AC1100 strain as a unique carbon source (Coeyne et al., 2004). Similarly, B. cepacia PCL3 uses carbofuran (2,3-dihydro -2,2-dimethylbenzofuran -7-il methylcarbamate), a wide spectrum insecticide used in agricultural activities to control insects and nematodes, as a unique carbon source (Plangklang and Reungsang, 2008). Plangklang and Reungnsang (2011) reported that the immobilized cells of B. cepacia PCL3 were more effective to reduce carbofuran half-life from 127 to 16 days compared to the free cells that took from 127 to 28 Degradation of pyrene, a polycyclic aromatic hydrocarbon (PAH), which is widely distributed in aquatic environments, predominantly occurs microbially through the activity of the 2,3-dioxygenase catechol enzyme (C23O) of B. cepacia (Chen et al., 2013).
A large amount of Burkholderia species are usually isolated from the rhizosphere, which makes them ideal for rhizoremediation strategies, that is, degradation of contaminants by rhizosphere bacteria. The genomic analysis of the degrading routes could eventually be useful to optimize the Burkholderia strains in the bioremediation processes, as well as to develop new or more efficient processes for the degradation of contaminants (O’Sullivan and Mahenthiralingam, 2005).
Human pathogenic Burkholderia. Some members of the Burkholderia genus are opportunistic pathogens in human beings. For example, B. pseudomallei and B. mallei are the causal agents of melioidosis and glanders, respectively (Wiersinga et al., 2006). These are animal and human severe diseases, endemic in southeast Asia and northern Australia, whose symptoms are confused with those of tuberculosis and pneumonia (Ulrich et al., 2004).
In 1984, Isles et al. reported the first case of infection by B. cepacia in patients with cystic fibrosis (CF). One year later, the second report confirmed that the infections caused by this bacterium were associated with CF (Tablan et al., 1985). Cystic fibrosis is a genetic disease associated with pancreatic insufficiency and airways infections (Anderson, 1938).
The cystic fibrosis transmembrane regulator (CFTR), a protein encoded by the CF gene (Riordan et al., 1989) acts as a chloride ion channel. People with mutations in the CF gene alleles have severe defects in chloride ion transportation and typically salty sweat. This defect causes sticky dehydrated mucosa in different ducts of the body such as female sexual ducts, and pancreatic and pulmonary ducts, which are prone to severe microbial colonization. During the disease, patients may suffer from bronchiolitis, atelectasis, hemoptysis, pneumothorax, fibrosis, respiratory failure, and eventually, death (Govan and Deretic, 1996). Chronic microbial colonization transmitted by the air causes pulmonary infection, which is the main cause of morbidity and mortality of CF patients (Gilligan, 1991). The pathogen is transmitted to humans through nosocomial infections, that is, by the multiplication of microorganisms within the body during hospitalization, and they may or may not have symptoms (Farías, 2008).
The typical CF pathogens are Staphylococcus aureus, Pseudomonas aeruginosa and Haemophilus influenzae, but there were also reports of non-glucose fermenting pathogens such as Stenotrophomonas maltophilia, Alcaligenes xylosoxidans, R. pickettii and B. gladioli (Burns et al., 1998). It is known that P. aeruginosa usually infects CF patients and that B. cepacia is the most lethal opportunistic pathogen (Govan and Deretic, 1996).
From 1984 to 1985, B. cepacia aggressiveness in CF cases was documented as “cepacia syndrome” due to the decease of many individuals in hospitals. In 1990, the strain called “type 12,” “ET12,” “cable pilus strain” or “type 2” was reported to prevail in individual populations with CF in Canada and the United Kingdom (Mahenthiralingam et al., 2008).
From 1993 to 2009, a study of 33 cases of bacteriemias caused by B. cepacia was conducted in Spain, 21 of which were detected in two outbreaks. The source of the first outbreak could not be identified but the source of the second outbreak was a moisturizing cream lot (Ibarguren et al., 2011).
Historically, in the United Kingdom, B. cenocepacia has been the most abundant BCC pathogen in CF. However, due to the strict control practices, its prevalence was reduced and replaced by B. multivorans as the new dominant pathogen. Thus, an epidemiological change took place, which also occurred in the United States (Mahenthiralingam et al., 2008).
To study the pathogenic potential of some Burkholderia symbiotic-mutualist strains, Angus et al. (2014) inoculated the Caenorhabditis elegans nematode and HeLa cells (of human cervical carcinoma). Based on their results, the authors concluded that there was an extremely low risk of opportunistic infections by symbiotic-mutualist bacteria such as B. tuberum. However, Mahenthiralingam et al. (2008) reported the presence of strains clonally identical to those of BCC that cause infection in natural environments. This fact causes concern about the use of these bacteria in agricultural activities, such as biofertilization, bioremediation or biological control (Table 3).
The analysis of 17 Burkholderia isolates from humans and from the environment confirmed that there is no phylogenetic subdivision to distinguish between beneficial and pathogenic strains (Peeters et al., 2016).
Figure 3 shows a model of the mutualistic and pathogenic symbiosis of Burkholderia sensu lato genus. There is a large bacterial pool to establish beneficial and deleterious relationships with plants, as well as antagonistic relationships with animals and human beings. It is uncertain how many more environmental burkhordelias could be pathogenic for humans, or how human pathogenic burkholderias can move from the agricultural environment to hospitals, and vice versa. Given this controversial situation, and considering the process of gene loss and gain, which occurs naturally in bacteria, the question remains: are environmental and pathogenic burkholderias the same?
Conclusions
Burkholderia sensu lato is a versatile and controversial genus. It is versatile because its species establish symbiotic-mutualistic relationships with plants, and symbiotic-pathogenic relationships with plants, animals, and human beings; and controversial because some environmental and plant pathogens can at the same time be pathogenic to humans. Actually, the number of nitrogen fixing and plant growth promoting species that were analyzed was too low to be able to conclude that they are of low risk to human health. Unfortunately, the recent analyses of Burkholderia from humans and from the environment do not allow to distinguish between beneficial and pathogenic strains. Although pathogenicity tests in vitro provide valuable information, they should be taken with reserve because the conditions in the field are different and changing. The condition of susceptibility in humans who handle these microorganisms should also be considered. The intermediate groups found between the two burkholderia lineages are one of the reasons to investigate the possible gene exchange between symbiotic-mutualistic and symbiotic-pathogenic burkholderias. It is therefore risky to generalize the idea that the environmental burkholderias are not pathogenic to humans. Recommending the use of the A-lineage species in agricultural activities, such as biofertilization or bioremediation, is unethical, unless there is experimental evidence that they are safe for humans and animals. Finally, as a precautionary measure, since 2003, the United States Environmental Protection Agency has restricted the use of B. cepacia.
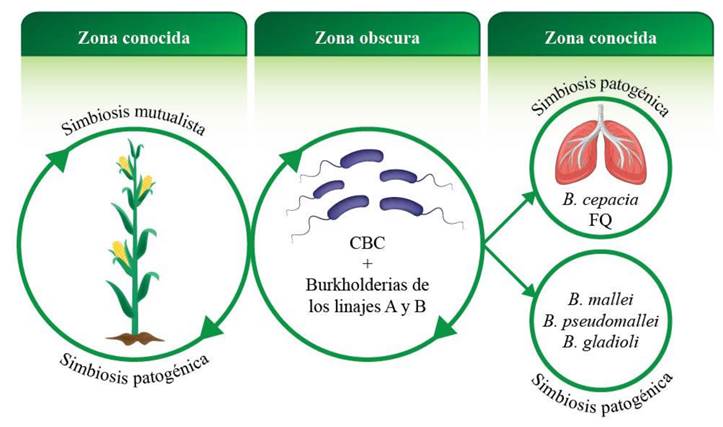
Figure 3 Model of the mutualistic and pathogenic symbiosis of the Burkholderia sensu lato genus. The circle in the center shows the Burkholderia species pool (BCC and burkholderias of lineages A and B). The arrows indicate a dynamic taxonomy with a continuous species reclassification. The circle on the left represents the group of burkholderias that establish mutualistic and/or pathogenic relationships with plants (the arrows highlight the constant movement from one condition to another). The circles on the right indicate the pathogenic relationships that the Burkholderia species establish with human beings and animals. The circle on the upper part highlights cystic fibrosis (CF), where P. cepacia was the most lethal pathogen for human beings. The circle on the lower part shows three species (B. mallei, B. pseudomalei and B. gladioli) pathogenic to human beings and/or animals which are not considered within the 22 BCC species (Table 1) and, therefore, not associated with CF. The circle on the left and the two on the right are well documented, but the one in the center is still dark, that is, that it is not known what other Burkholderia species could be pathogenic to humans and animals. The transit route between the circle in the center and those on the right is also unclear. BCC= Burkholderia cepacia complex.
Literatura Citada
Anderson, DH. 1938. Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathologic study. The American Journal of Diseases of Children 56:344-399. http://dx.doi.org/10.1001/archpedi.1938.01980140114013 [ Links ]
Angus, AA., Agapakis, CM., Fongm, S., Yerrapragada, S., Estrada-de los Santos, P., Yang, P., Song, N., Kano, S., Caballero-Mellado, J., de Faria, SM., Dakora, FD., Weinstock, G., and Hirsch, AM. 2014. Plant-associated symbiotic Burkholderia species lack hallmark strategies required in mammalian pathogenesis. Public Library of Science ONE 9: e83779. http://dx.doi.org/10.1371/journal.pone.0083779 [ Links ]
Angus, AA., Lee, A., Lum, MR., Shehayeb, M., and Hessabi, R. 2013. Nodulation and effective nitrogen fixation of Macroptilium atropurpureum (siratro) by Burkholderia tuberum, a nodulating and plant growth promoting beta-proteobacterium, are influenced by environmental factors. Plant and Soil 369:543-562. http://dx.doi.org/10.1007/s11104-013-1590-7 [ Links ]
Bevivino, A., Sarrocco, S., Dalmastri, C., Tabacchioni, S., Cantale, C., and Chiarini, L. 1998. characterization of a free-living maize-rhizosphere population of Burkholderia cepacia: effect of seed treatment on disease suppression and growth promotion of maize. FEMS Microbiology Ecology 27:225-237. http://dx.doi.org/10.1016/S0168-6496(98)00069-5 [ Links ]
Bianciotto, V., Lumini, E., Bonfante, P., and Vandamme, P. 2003. ‘Candidatus Glomeribacter gigasporarum’ gen nov., sp. nov., an endosymbiont of arbuscular mycorrhizal fungi. International Journal of Systematic and Evolutionary Microbiology 53:121-124. http://dx.doi.org/10.1099/ijs.0.02382-0 [ Links ]
Bianciotto V, Lumini E, Lanfranco L, Minerdi D, Bonfante P, and Perotto S. 2000. Detection and identification of bacterial endosymbionts in arbuscular mycorrhizal fungi belonging to the family Gigasporaceae. Applied and Environmental Microbiology 66:4503-4509. http://dx.doi.org/10.1128/aem.66.10.4503-4509.2000 [ Links ]
Burkhead, KD., Schisler, DA., and Slininger, PJ. 1994. Pyrrolnitrin production by biological control agent Pseudomonas cepacia B37w in culture and in colonized wounds of potatoes. Applied and Environmental Microbiology 60:2031-2039. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC201598/pdf/aem00023-0337.pdf [ Links ]
Burkholder, W. 1950. Sour skin, a bacterial rot of onion bulbs. Phytopathology 64:468-475. https://www.cabdirect.org/cabdirect/abstract/19501101355 [ Links ]
Burns, JL., Emerson, J., Stapp, JR., Yim, DL., Krzewinski, J., Louden, L., Ramsey, BW., and Clausen, CR. 1998. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clinical Infectious Diseases 27:158-163. http://dx.doi.org/10.1086/514631 [ Links ]
Caballero-Mellado, J., Martínez-Aguilar, L., Pardes-Valdez, G., and Estrada-de los Santos, P. 2004. Burkholderia unamae sp. nov., an N2-fixing rhizospheric and endophitic species. International Journal of Systematic and Evolutionary Microbiology 54:1165-1172. http://dx.doi.org/10.1099/ijs.0.02951-0 [ Links ]
Cartwright, DK., Chilton, WS., and Benson, DM. 1995. Pyrrolnitrin and phenazine production by Pseudomonas cepacia, strain 5.5B, a biocontrol agent of Rhizoctonia solani. Applied Microbiology and Biotechnology 43:211-16. https://link.springer.com/content/pdf/10.1007%2FBF00172814.pdf [ Links ]
Chen, K., Zhu, Q., Qian, Y., Song, Y., Yao, J., and Choi, MMF. 2013. Microcalorimetric investigation of the effect of non-ionic surfactant on biodegradation of pyrene by PAH-degrading bacteria Burkholderia cepacia. Ecotoxicology and Environmental Safety 98:361-367. http://dx.doi.org/10.1016/j.ecoenv.2013.08.012 [ Links ]
Chen, WM., de Faria, SM., James, EK., Elliott, GN., Lin, K., Chou, JH., Sheu, SY., Cnockaert, M., Sprent, JI., and Vandamme, P. 2007. Burkholderia nodosa sp. nov., isolated from root nodules of the woody Brazilian legumes Mimosa bimucronata and Mimosa scabrella. International Journal of Systematic and Evolutionary Microbiology 57:1055-1059. http://dx.doi.org/10.1099/ijs.0.64873-0 [ Links ]
Chiarini, L., Bevivino, A., Dalmastri, C., Tabacchioni, S., and Visca, P. 2006. Burkholderia cepacia complex species: health hazards and biotechnological potential. Trends in Microbiology 14:277-286. http://dx.doi.org/10.1016/j.tim.2006.04.006 [ Links ]
Chun, H., Choi, O., Goo, E., Kim, N., Kim, H., Kang, Y., Kim, J., Moon, JS. & Hwang, I. 2009. The quorum sensing-dependent gene katG of Burkholderia glumae is important for protection from visible light. US: Journal of Bacteriology, 191:4152-4157. http://dx.doi.org/:10.1128/JB.00227-09 [ Links ]
Coeyne, T., Henry, D., Speert, DP. and Vandamme, P. 2004. Burkholderia phenoliruptrix sp. nov., to accommodate the 2,4,5trichlorophenoxyacetic acid and halophenol-degrading strain AC1100. Systematic and Applied Microbiology 27: 623-627. http://dx.doi.org/:10.1078/0723202042369992 [ Links ]
Coenye, T., Mahenthiralingam, E., Henry, D., Lipuma, JJ., Laevens, S., Gillis, M., Speert, DP., and Vandamme, P. 2001. Burkholderia ambifaria sp. nov., a novel member of the Burkholderia cepacia complex including biocontrol and cystic fibrosis-related isolates. International Journal of Systematic and Evolutionary Microbiology 51:1481-1490. http://dx.doi.org/10.1099/00207713-51-4-1481 [ Links ]
De los Santos-Villalobos, S., Barrera-Galicia, GC., Miranda-Salcedo, MA., and Pena-Cabriales, JJ. 2012. Burkholderia cepacia XXVI siderophore with biocontrol capacity against Colletotrichum gloeosporioides. World Journal of Microbiology and Biotechnology 28:2615-2623. http://dx.doi.org/10.1007/s11274-012-1071-9 [ Links ]
De Smet, B., Mayo, M., Peeters, C., Zlosnik, JE., Spilker, T., Hird, TJ., LiPuma, JJ., Kidd, TJ., Kaestli, M., Ginther, JL., Wagner, DM., Keim, P., Bell, SC., Jacos, JA., Currie, BJ., and Vandamme, P. 2015. Burkholderia stagnalis sp. nov. and Burkholderia territorii sp. nov., two novel Burkholderia cepacia complex species from environmental and human sources. International Journal of Systematic and Evolutionary Microbiology 65: 2265-2271. http://dx.doi.org/10.1099/ijs.0.000251 [ Links ]
Dobritsa, AP., and Samadpour, M. 2016. Transfer of eleven species of the genus Burkholderia to the genus Paraburkholderia and proposal of Caballeronia gen. nov. to accommodate twelve species of the genera Burkholderia and Paraburkholderia. International Journal of Systematic and Evolutionary Microbiology 66: 2836-2846. http://dx.doi.org/10.1099/ijsem.0.001065 [ Links ]
Estrada-de los Santos, P., Martínez-Aguilar, L., Vinuesa, P., Hirsch, AM., and Caballero-Mellado, J. 2013. Phylogenetic analysis of Burkholderia species by Multilocus Sequence Analysis. Current Microbiology 67:51-60. http://dx.doi.org/10.1007/s00284-013-0330-9 [ Links ]
Estrada-de los Santos, P., Rojas-Rojas, FU., Tapia-García, EY., Vásquez-Murrieta, MS., and Hirsch, AM. 2015. To split or not to split: an opinion on dividing the genus Burkholderia. Annals of Microbiology 66:1303-1314. http://dx.doi.org/10.100 7/s13213-015-1183-1 [ Links ]
Farias, SJA. 2008. Burkholderia cepacia (B. cepacia). Nuevo patógeno de infecciones nosocomiales. Serie de casos clínicos. Enfermedades Infecciosas y Microbiología Clínica 28:19-23. https://www.medigraphic.com/pdfs/micro/ei-2008/ei081d.pdf [ Links ]
Ferrer, M., Golyshin, P. and Timmis, KN. 2003. Novel maltotriose esters enhance biodegradation of Aroclor 1242 by Burkholderia cepacia LB400. World Journal of Microbiology and Biotechnology 19: 637-643. https://doi.org/10.1023/A:1025124019986 [ Links ]
Garrity, G., Staley, JT., Boone, DR., De Vos, P., Goodfellow, M., Rainey, F A., and Schleifer, KH. 2006. Bergey’s Manual of Systematic Bacteriology: Volume Two: The Proteobacteria. D. J., Brenner, & N. R., Krieg (Eds.). Springer Science & Business Media. https://www.springer.com/gp/book/9780387241449 [ Links ]
Ghosh, R., Barman, S., Mukherjee, R., and Mandal, L. 2016. Role of solubilizing Burholderia spp. for successful and growth promotion of Lycopodium cernuum L. (Lycopodiaceae) in lateritic belt of Birbhum district of West Bengal, India. Microbial Research 183: 80-91. http://dx.doi.org/10.1016/j.micres.2015.11.011 [ Links ]
Gilligan, PH. 1991. Microbiology of airway disease in patients with cystic fibrosis. Clinical Microbiological Reviews 4:35-51. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC358177/pdf/cmr00042-0051.pdf [ Links ]
Gillis, M., Vanvan, T., Bardin, R., Goor, M., Hebbar, P., Willems, A., Segers, P., and Kersters, K. 1995. Polyphasic taxonomy in the genus Burkholderia leading to an emended description of the genus and proposition of Burkholderia vietnamiensis sp-nov for N-2-fixing isolates from rice in Vietnam. International Journal of Systematic and Evolutionary Microbiology 45:274-289. https://www.microbiologyresearch.org/docserver/fulltext/ijsem/45/2/ijs-45-2-274.pdf?expires=1570735297&id=id&accname=guest&checksum=A3753345BCC75CCA575AEFC833B09F8F [ Links ]
Govan, JRW., and Deretic, V. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiological Reviews 60:539-574. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC239456/pdf/600539.pdf [ Links ]
Gyaneshwar, P., Kumar, GN., and Parekh, L. 2002. Role of soil microorganisms in improving P nutrition of plants. Plant and Soil 245:83-93. https://link.springer.com/content/pdf/10.1023%2FA%3A1020663916259.pdf [ Links ]
Heungens, K., and Parke, JL. 2000. Zoospore homing and infection events: effects of the biocontrol bacterium Burkholderia cepacia AMMDRI on two oomycete pathogens of pea (Pisum sativum L.). Applied and Environmental Microbiology 66:5192-5200. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC92443/pdf/am005192.pdf [ Links ]
Huang, Y., and Wong, PTW. 1998. Effect of Burkholderia (Pseudomonas) cepacia and soil type on the control of crown rot in wheat. Plant and Soil 203:103-108. https://link.springer.com/content/pdf/10.1023%2FA%3A1004377801490.pdf [ Links ]
Ibarguren, PM., Cobos-Trigueros, N., Soriano, A., Martínez, JA., Zboromyrska, Y., Almela, M., y Mensa, J. 2011. Bacteriemias por Burkholderia cepacia: análisis prospectivo de 33 episodios. Revista Española de Quimioterapia 24:209-212. https://seq.es/seq/0214-3429/24/4/ibarguren.pdf [ Links ]
Isles, A., Maclusky, I., Corey, M., Gold, R., Prober, C., Fleming, P., and Levison, H. 1984. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. Journal Pediatrics 104: 206-210. http://dx.doi.org/10.1016/S0022-3476(84)80993-2 [ Links ]
Jacobs, JL., Fasi, AC., Ramette, A., Smith, JJ., Hammerschmidt, R., and Sundin, GW. 2008. Identification and onion pathogenicity of Burkholderia cepacia Complex isolates from the onion rhizosphere and onion field soil. Applied and Environmental Microbiology 74:3121-3129. http://dx.doi.org/10.1128/AEM.01941-07 [ Links ]
Ham, JH., Melanson, RA. & Rush, MC.. 2011. Burkholderia glumae: next major pathogen of rice? Molecular Plant Pathology 12: 329-339 http://dx.doi.org/:10.1111/j.1364-3703.2010.00676.x [ Links ]
Jiao, Y., Yoshihara, T., Ishikuri, S., Uchino, H., and Ichihara, A. 1996. Structural identification of cepaciamide A, a novel fungitoxic compound from Pseudomonas cepacia D-202. Tetrahedron Letters 37:1039-1042. http://dx.doi.org/10.1016/0040-4039(95)02342-9 [ Links ]
Jin, Y., Zhou, J., Zhou, J., Hu, M., Zhang, Q., Kong, Na., Ren, H., Liang, L. and Yue, J. 2020. Genome-based classification of Burkholderia cepacia complex provides new insight into its taxonomic status. Biology Direct 15 (6): 1-14. https://doi.org/10.1186/s13062-020-0258-5 [ Links ]
Kang, Y., Carlson, R., Tharpe, W., and Schell, MA. 1998. Characterization of genes involved in biosynthesis of a novel antibiotic from Burkholderia cepacia BC11 and their role in biological control of Rhizoctonia solani. Applied and Environmental Microbiology 64:3939-3947. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC106582/pdf/am003939.pdf [ Links ]
Keith, L. y Thammakijjawat, P. 2019. Detection of Burkholderia glalioli in Orchids. https://doi.org/10.1094/9780890545416.048 [ Links ]
Kim, J., Kang, Y., Choi, O., Jeong, Y., Jeong, Y., Jeong, J-E., Lim, JY., Kim, M., Moon, JS., Suga, H. & Hwang, I. 2007. Regulation of polar flagellum genes is mediated by quorum sensing and FlhDC in Burkholderia glumae. Molecular Microbiology, 64(1), pp.165-179. http://dx.doi.org/:10.1111/j.1365-2958.2007.05646.x [ Links ]
Knudsen, GR., and Spurr, HW Jr. 1987. Field persistence and efficacy of five bacterial preparations for control of peanut leaf spot. Plant Disease 71:442-445. https://www.apsnet.org/publications/PlantDisease/BackIssues/Documents/1987Articles/PlantDisease71n05_442.PDF [ Links ]
Larsen, GY., Stull, TL., and Burns, JL. 1993. Marked phenotypic variability in Pseudomonas cepacia isolated from a patient with cystic fibrosis. Journal of Clinical Microbiology 31:788-792. https://jcm.asm.org/content/jcm/31/4/788.full.pdf [ Links ]
Lee, CH., Kim, S., Hyun, B., Suh, JW., and Yon, C., 1994. Cepacidine A, a novel antifungal antibiotic produced by Pseudomonas cepacia. I. Taxonomy, production, isolation and biological activity. The Journal of Antibiotics (Tokyo) 47:1402-1405. http://dx.doi.org/10.7164/antibiotics. 47.1402 [ Links ]
Levy, A., Chang, BJ., Abbott, LK., Kuo, J., Harnett, G., and Inglis, TJJ. 2003. Invasion of spores of the arbuscular mycorrhizal fungus Gigaspora decipiens by Burkholderia spp. Applied and Environmental Microbiology 69:6250-6256. http://dx.doi.org/10.1128/aem.69.10.6250-6256.2003 [ Links ]
Mahenthiralingam, E., Baldwin, A., and Dowson, CG. 2008. Burkholderia cepacia complex bacteria: opportunistic pathogens with important natural biology. Journal of Applied Microbiology 104:1539-1551. http://dx.doi.org/10.1111/j.1365-2672.2007.03706.x [ Links ]
Martin, FN., and Loper, JE. 1999. Soilborne plant diseases caused by Pythium spp.: ecology, epidemiology, and prospects for biological control. Critical Reviews in Plant Sciences 18:111-81. http://dx.doi.org/10.1080/07352689991309216 [ Links ]
Martínez-Aguilar, L., Salazar-Salazar, C., Díaz-Méndez, R., Caballero-Mellado, J., Hirsch, AM., Vásquez-Murrieta, MS., and Estrada-de los Santos, P. 2013. Burkholderia caballeronis sp. nov., a nitrogen fixing species isolated from tomato (Lycopersicon esculentum) with the ability to effectively nodulate Phaseolus vulgaris. Antonie van Leeuwenhoek 104:1063-1071. http://dx.doi.org/10.1007/s10482-013-0028-9 [ Links ]
Minerdi, D., Fani, R., Gallo, R., Boarino, A., and Bonfante, P. 2001. Nitrogen fixation genes in an endosymbiotic Burkholderia strain. Applied and Environmental Microbiology 67:725-732. http://dx.doi.org/10.1128/AEM.67.2.725-732.2001 [ Links ]
Mirghasempour, S.A., Huang, S., Xie, G. L. 2018. First report of Burkholderia gladioli causing rice panicle blight and grain discoloration in China. https://doi.org/10.1094/PDIS-05-18-0758-PDN [ Links ]
Moon, SS., Kang, PM., Park, KS., and Kim, CH. 1996. Plant growth promoting and fungicidal 4-quinolinones from Pseudomonas cepacia. Phytochemistry 42:365-368. http://dx.doi.org/10.1016/0031-9422(95)00897-7 [ Links ]
Nandakumar, R., Shahjahan, AK., Yuan, XL., Dickstein, ER., Groth, DE., Clark, CA., Cartwright, RD. & Rush, MC. 2009. Burkholderia glumae and B. gladioli cause bacterial panicle blight in rice in the southern United States. US: Plant Disease 93: 896-905. http://dx.doi.org/:10.1094/PDIS-93-9-0896 [ Links ]
Oren, A., and Garrity, GM. 2015b. List of new names and new combinations previously effectively, but not validly, published. International Journal of Systematic and Evolutionary Microbiology 65: 2777-2783. http://dx.doi.org/10.1099/ijsem.0.000464 [ Links ]
O´Sullivan, LA., and Mahenthiralingam, E. 2005. Biotechnological potential within the genus Burkholderia. Letters in Applied Microbiology 41:8-11. http://dx.doi.org/10.1111/j.1472-765X.2005.01758.x [ Links ]
Parke, JL., and Gurian-Sherman, D. 2001. Diversity of the Burkholderia cepacia complex and implications for risk assessment of biological control strains. Annual Review of Phytopathology 39:225-258. http://dx.doi.org/10.1146/annurev.phyto.39.1.225 [ Links ]
Parker, WL., Rathnum, ML., Seiner, V., Trejo, WH., Principe, PA., and Sykes, RB. 1984. Cepacin A and cepacin B, two new antibiotics produced by Pseudomonas cepacia. The Journal of Antibiotics 37:431-40. http://dx.doi.org/10.7164/antibiotics.37.431 [ Links ]
Parra-Cota, FI., Peña-Cabriales, JJ., de los Santos-Villalobos, S., Martínez-Gallardo, NA., and Délano-Frier, JP. 2014. Burkholderia ambifaria and B. caribensis promote growth and Increase yield in rain amaranth (Amaranthus cruentus and A. hypochondriacus) by improving plant nitrogen uptake. Public Library of Science ONE 9:e88094. http://dx.doi.org/10.1371/journal.pone.0088094 [ Links ]
Partida-Martinez, LP., Groth, I., Schmitt, I., Richter, W., Roth, M., and Hertweck, C. 2007. Burkholderia rhizoxinica sp. nov. and Burkholderia endofungorum sp. nov., bacterial endosymbionts of the plant-pathogenic fungus Rhizopus microsporus. International Journal of Systematic and Evolutionary Microbiology 57:2583-2590. http://dx.doi.org/10.1099/ijs.0.64660-0 [ Links ]
Peeters, C., Meier-Kolthoff, JP., Verheyde, B., De Brandt E, Cooper VS, and Vandamme, P. 2016. Phylogenomic study of Burkholderia glathei-like organisms, proposal of 13 novel Burkholderia species and emended descriptions of Burkholderia sordidicola, Burkholderia zhejiangensis, and Burkholderia grimmiae. Frontiers in Microbiology. 7:1-19. http://dx.doi.org/10.3389/fmicb.2016.00877 [ Links ]
Pérez, C. y Saavedra, E. 2011. Avances en el manejo integrado de la bacteria Burkholderia glumae en el cultivo de arroz en el Caribe colombiano. Colombia: Revista Colombiana de Ciencia Animal 3(1): 111-124. https://doi.org/10.24188/recia.v3.n1.2011.344 [ Links ]
Plangklang, P. and Reungsang, A. 2008. Effects of rhizosphere remediation and bioaugmentation on carbofuran removal from soil. World Journal of Microbiology & Biotechnology 24:983-989. http://dx.doi.org/10.1007/s11274-007-9562-9 [ Links ]
Plangklang, P., and Reungsang, A. 2011 Bioaugmentation of carbofuran residues in soil by Burkholderia cepacia PCL3: A small-scale field study. International Biodeterioration & Biodegradation 65:902-905. http://dx.doi.org/10.1016/j.ibiod.2011.02.011 [ Links ]
Quesada-González, A., García-Santamaría, F. 2014. Burkholderia glumae en el cultivo de arroz en Costa Rica. Agronomía Mesoamericana 25(2):371-381. https://www.redalyc.org/pdf/437/43731480015.pdf [ Links ]
Ramírez-Rojas, S., Osuna-Canizalez, FJ., García-Pérez, F., Canul-Ku, J., Palacios-Talavera, A., Hernández-Romano, J., Ornelas-Ocampo, K. y Landa-Salgado, P. 2016. Identificación molecular de bacterias asociadas a plantas ornamentales producidas in vitro. Revista Mexicana de Fitopatología 34:173-183. http://dx.doi.org/10.18781/R.MEX.FIT.1511-3 [ Links ]
Riordan, JR., Rommens, JM., Kerem, BS., Alon, N., Rozmahel, R., Grzelczak, K., Zielenski, J., Lok, J., Plasic, J., Chou, JL., Drumm, ML., Ianuzzi, MC., Collins, FS., and Tsui, LC. 1989. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245:1066-1073. http://dx.doi.org/10.1126/science.2475911 [ Links ]
Rojas-Rojas, FU., López-Sánchez, D., Meza-Radilla, G., Méndez-Canarios, A., Ibarra, JA. y Estrada-de los Santos, P. 2019. El controvertido complejo Burkholderia cepacia, un grupo de especies promotoras del crecimiento vegetal y patógenas de plantas, animales y humanos. Revista Argentina de Microbiología 51 (1): 84-92. http://dx.doi.org/10.1016/j.ram.2018.01.002 [ Links ]
Sánchez-Yáñez, JM., Villegas Moreno, J., Vela-Muzquiz, GR., y Márquez-Benavides, L. 2014. Respuesta del garbanzo (Cicer arietinum L.) a la inoculación con Azotobacter vineladii y Burkholderia cepacia a dosis reducida de fertilizante nitrogenado. Scientia Agropecuaria 5:115-120. http://www.scielo.org.pe/pdf/agro/v5n3/a01v5n3.pdf [ Links ]
Sayler, RJ., Cartwright, RD. & Yang, Y. 2006. Genetic characterization and real-time PCR detection of Burkholderia glumae, a newly emerging bacterial pathogen of rice in the United States. US: Plant Disease 90 (5): 603-610. http://dx.doi.org/:10.1094/PD-90-0603 [ Links ]
Sawana, A., Adeolu, M., y Gupta, RS. 2014. Molecular signatures and phylogenomic analysis of the genus Burkholderia: proposal for division of this genus into the emended genus Burkholderia containing pathogenic organisms and a new genus Paraburkholderia gen. nov. harboring environmental species. Frontiers in Genetics 5: 1-22. http://dx.doi.org/10.3389/fgene.2014.00429 [ Links ]
Seo, YS., Lim, J., Choi, BS., Kim, H., and Goo, E. 2011. Complete genome sequence of Burkholderia gladiolii BSR3. Journal of Bacteriology 193:3149. http://dx.doi.org/10.1128/JB.00420-11 [ Links ]
Shahjahan, AKM., Rush, MC., Groth, D., and Clark, C. 2000. Panicle blight. Recent research points to a bacterial cause. Rice Journal 15:26-29. http://www.ricejournal.com/april2000 [ Links ]
Shields, MS., Reagin, MJ., Gerger, RR., Campbell, R. and Somerville, C. 1995. TOM, a new aromatic degradative plasmid from Burkholderia (Pseudomonas) cepacia G4. Applied Environmental Microbiology 61: 1352-1356. https://pubmed.ncbi.nlm.nih.gov/7538275/ [ Links ]
Singh, RK., Malik, N., and Singh, S. 2013. Impact of rhizobial inoculation and nitrogen utilization in plant growth promotion of maize (Zea mays L.). Nusantara Bioscience 5:8-14. https://doi.org/10.13057/nusbiosci/n050102 [ Links ]
Sokol, PA., Darling, P., Woods, DE., Mahenthiralingam, E., and Kooi, C. 1999. Role of ornibactin biosynthesis in the virulence of Burkholderia cepacia: characterization of pvdA, the gene encoding L-ornithine N (5)-oxygenase. Infection and Immunity 67:4443-55. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC96763/pdf/ii004443.pdf [ Links ]
Stoyanova, M., Georgieva, L., Moncheva, P., and Bogatzevska, N. 2013. Burkholderia gladioli and Pseudomonas marginalis pathogens of Leucojum aestivum. Biotechnology & Biotechnological Equipment 27:4069-4073. http://dx.doi.org/10.5504/BBEQ.2012.0139 [ Links ]
Suzuki, F., Sawada, H., Azegami, K. & Tsuchiya, K. 2004. Molecular characterization of the tox operon involved in toxoflavin biosynthesis of Burkholderia glumae. Journal of General Plant Pathology 70: 97-107. https://doi.org/10.1007/s10327-003-0096-1 [ Links ]
Tablan, OC., Chorba, TL., Schidlow, DV., White, JW., Hardy, KA., Gilligan, PH., Morgan, WM., and Carson, LA. 1985. Pseudomonas cepacia colonization in patients with cystic fibrosis: risk factors and clinical outcome. The Journal of Pediatrics 107:382-387. http://dx.doi.org/10.1016/s0022-3476(85)80511-4 [ Links ]
Ulrich, RL., DeShazer, D., Hines, HB., and Jeddeloh, JA. 2004. Quorum sensing: a transcriptional regulatory system involved in the pathogenicity of Burkholderia mallei. Infection and Immunity 72:6589-6596. http://dx.doi.org/10.1128/IAI.72.11.6589-6596.2004 [ Links ]
Van Borm, S., Buschinger, A., Boomsma, JJ., and Billen, J. 2002. Tetraponera ants have gut symbionts related to nitrogen-fixing root-nodule bacteria. Proceedings of the Royal Society of London B: Biological Sciences 269:2023-2027. http://dx.doi.org/10.1098/rspb.2002.2101 [ Links ]
Van Oevelen, S., De Wachter, R., Vandamme, P., Robbrecht, E., and Prinsen, E. 2002. Identification of the bacterial endosymbionts in leaf galls of Psychotria (Rubiaceae, angiosperms) and proposal of ‘Candidatus Burkholderia kirkii’ sp. nov. International Journal of Systematic and Evolutionary Microbiology 52:2023-2027. http://dx.doi.org/10.1099/00207713-52-6-2023 [ Links ]
Vandamme, P., Henry, D., Coenye, T., Nzula, S., Vancanneyt, M., Lipuma, JJ., Speert, DP., and Govan, JR. 2002. Burkholderia anthina sp. nov. Burkholderia pyrrocinia, two additional Burkholderia cepacia complex bacteria, may confound results of new molecular diagnostic tools. FEMS Immunology & Medical Microbiology 33:143-149. http://dx.doi.org/10.1111/j.1574-695X.2002.tb00584.x [ Links ]
Vandamme, P., Holmes, B., Coenye, T., Goris, J., Mahenthiralingam, E., Lipuma, JJ., and Govan, JR. 2003. Burkholderia cenocepacia sp. nov- a new twist to an old story. Research in Microbiology 154:91-96. http://dx.doi.org/10.1016/S0923-2508(03)00026-3 [ Links ]
Vandamme, P., Holmes, B., Vancanneyt, M., Coenye, T., Hoste, B., Coopman, R., Revets, H., and Lauwers, S. 1997. Occurrence of multiple genomovars of Burkholderia cepacia in cystic fibrosis patients and proposal of Burkholderia multivorans sp. nov. International Journal of Systematic Bacteriology 47:1188-1200. http://dx.doi.org/10.1099/00207713-47-4-1188 [ Links ]
Vandamme, P., Mahenthiralingam, E., Holmes, B., Coenye, T., Hoste, B., De Vos, P., Henry, D., and Speert, DP. 2000. Identification and population structure of Burkholderia stabilis sp. nov. (Formerly Burkholderia cepacia genomovar IV). Journal of Clinical Microbiology 38:1042-1047. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC86333/pdf/jm001042.pdf [ Links ]
Vermis, K., Coenye, T., Lipuma, JJ., Mahenthiralingam, E., Nelis, HJ., and Vandamme, P. 2004. Proposal to accommodate Burkholderia cepacia genomovar VI as Burkholderia dolosa sp. nov. International Journal of Systematic and Evolutionary Microbiology 54:689-691. http://dx.doi.org/10.1099/ijs.0.02888-0 [ Links ]
Vermis, K., Coenye, T., Mahenthiralingam, E., Nelis, HJ., and Vandamme, P. 2002. Evaluation of species-specific recA-based PCR tests for genomovar level identification within the Burkholderia cepacia complex. Journal of Medical Microbiology 51:937-940. http://dx.doi.org/10.1099/0022-1317-51-11-937 [ Links ]
Vidaver, AK., Carlson, RR. 1978. Leaf spot of field corn caused by Pseudomonas andropogonis. Plant Disease Report 62:213-216. http://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1004&context=bpdfpub [ Links ]
Weber, CF., and King, GM. 2017. Volcanic soils as sources of novel CO-oxidizing Paraburkholderia and Burkholderia: Paraburkholderia hiiakae sp. nov., Paraburkholderia metrosideri sp. nov., Paraburkholderia paradisi sp. nov., Paraburkholderia peleae sp. nov., and Burkholderia alpina sp. nov. a member of the Burkholderia cepacia Complex. Front. Microbiol. 8: 1-10. 207. http://dx.doi.org/10.3389/fmicb.2017.00207 [ Links ]
Wiersinga, WJ., van der Poll, T., White, NJ., Day, NP., and Peacock, SJ. 2006. Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nature Reviews in Microbiology 4:272-282. http://dx.doi.org/10.1038/nrmicro1385 [ Links ]
Xin G, Zhang G, Kang JW, Staley JT, and Doty SL. 2009. A diazotrophic, indole-3-acetic acid-producing endophyte from wild cottonwood. Biology and Fertility of Soils. http://dx.doi.org/10.1007/s00374-009-0377-8 [ Links ]
Yabuuchi, E., Kosako, Y., Oyaizu, H., Yano, I., Hotta, H., and Hashimoto, Y. 1992. Proposal of Burkholderia gen nov. and transfer of seven species of the genus Pseudomonas homology group II to the new genus, with the type species Burkholderia cepacia (Palleroni and Holmes, 1981) comb. nov. Microbiology and Immunology 36:1251-1275. http://dx.doi.org/10.1111/j.1348-0421.1992.tb02129.x [ Links ]
Yabuuchi, E., Kosako, Y., Yano, I., Hotta, H., and Nishiuchi, Y. 1995. Transfer of two Burkholderia and an Alcaligenes species to Ralstonia gen nov. proposal of Ralstonia pickettii (Ralston, Palleroni and Doudoroff, 1973) comb. nov., Ralstonia solanacearum (Smith, 1896) comb. nov. Ralstonia eutropha (Davis, 1969) comb. nov. Microbiology and Immunology 39:897-904. http://dx.doi.org/10.1111/j.1348-0421.1995.tb03275.x [ Links ]
Received: April 28, 2020; Accepted: June 04, 2020











 texto en
texto en