Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Educación química
versión impresa ISSN 0187-893X
Educ. quím vol.20 no.4 Ciudad de México oct. 2009
Cómo se experimenta
"Modernization" of the Chemistry Education Process: Do People Still Perform Real Experiments?
Vladimir M. Petruševski,1 Marina Stojanovska,1 Bojan Šoptrajanov2
1 Institute of Chemistry, Faculty of Natural Sciences and Mathematics, Ss. Cyril & Methodius University, Arhimedova 5, 1000 Skopje, Republic of Macedonia.
2 Macedonian Academy of Sciences and Arts, Bul. Krste Misirkov 2, 1000 Skopje, Republic of Macedonia.
Recibido: 11 de noviembre de 2008;
aceptado: 17 de febrero de 2009.
Abstract
During the last two decades, numerous attempts have been made to "modernize" science education, chemistry education in particular. Microscale experiments, such as projected experiments, have become favorites of many educators and instructors. Although these experiments might be useful for hands-on approach or for quick demonstrations in large lecture rooms,educators often overlook the shortcomings. Those whose wish is to "modernize"chemistry/ science education often use modern equipment that does not explain the science phenomena as well as the traditional experiment. Therefore, these microscale experiments sometimes distort the information gathered—information that is easily obtained by the macroscopic—or traditional—approach. The question arises: do we, occasionally, modernize just to be modern? This paper discusses two groups of examples: (1) those where the modern (in this case the microscale) approach is a step in the right direction and (2) examples where "modernization" appears to be a step backwards, like insisting on microscale experiments where they completely fail, or using projectors and movies instead of performing experiments in vivo.
Keywords: chemistry, demonstrations, experiments, microscale, modernization, overhead.
Resumen
Durante las últimas dos décadas se han hecho numerosos intentos de "modernizar" la educación en ciencias, en particular la educación química. Los experimentos de "microescala" se han vuelto los favoritos de muchos educadores. Aunque estos experimentos pueden ser útiles para un enfoque de "hacer con las manos" o para demostraciones rápidas en grandes salones de clase, los educadores a menudo dejan pasar sus limitaciones.
Aquellos cuyo deseo es "modernizar" la educación en ciencias/química utilizan a menudo equipo moderno que no explica tan bien como el experimento tradicional el fenómeno científico. Por lo tanto, estos experimentos en microescala algunas veces distorsionan la información reunida —información que puede obtenerse fácilmente por el enfoque macroscópico tradicional. La pregunta que surge es: ¿ocasionalmente nos modernizamos sólo por modernizar? Este trabajo discute dos tipos de ejemplo: (1) Aquellos en los que el enfoque modernizador (en el caso de microescala) es un paso en la dirección correcta y (2) ejemplos en los que la modernización es un paso hacia atrás, como insistir en experimentos de micro-escala que fallan por completo, o emplear proyectores o películas en lugar de realizar experimentos in vivo.
Introduction
Lecture demonstrations and experiments are important in the chemistry education process, and not surprisingly, a number of textbooks and monographs have been devoted to this subject (Fowles, 1959; Summerlin & Ealy, 1988; Summerlin, Borgford & Ealy, 1987; Shakhashiri, 1983; Shakhashiri, 1985; Shakhashiri, 1989; Shakhashiri, 1992). Hands-on experiments support the education process and rely not only on mental concepts, but also on the "use" of all five senses.
The last two decades reveal a pronounced trend of performing experiments on a microscale, often based on the use of purposely built equipment and small amounts of chemicals (Hugerat & Schwarz, 2008; Ibanez, Lopez-Mejia & Echevarria-Eugui, 2007; Moran-Moran & Hernandez-Esparza, 2004, and the references therein). Since 1987, the overhead has been used as an aid during chemistry lectures (Kolb, 1987). These "innovations" have become a standard approach, and in some cases, "a must." In fact, one of the criteria for acceptance of a manuscript to the Journal of Chemical Education is whether the offered demonstration can be projected.
There is no doubt that microscale experiments may often have some real advantages. However, a question crops at this point: is it really important to use an overhead even when successful classical demonstrations can be performed? Although these microscale experiments are safer and less expensive, are they the only valuable and acceptable approach? Being among the people that devoted themselves to the study of chemistry education (Petruševski, Monković & Ivanovski, 2006;Petruševski,Monković& šoptrajanov,2007;Petruševski, Stojanovska & šoptrajanov, 2007, and the references therein), we feel we could say a few words that will help to possibly answer the above question.
Historical Details
The first educator to use an overhead, it seems, was the famous organic chemistry textbook writer Carl Noller during his optical activity demonstrations (Noller, 1949). Two years later Slabaugh adapted the overhead for seven well-known classical demonstrations (Slabaugh, 1951). These demonstrations included activity series for metals, electrolysis of water, kinetic theory of gases, action of metal couples, relative strength of acids, optical activity demonstrations, and electrochemical cells demonstrations. Seven years later, Keenan presented more physical chemistry experiments that covered several topics in electrochemistry and gas laws (Keenan, 1958).
Hundreds of experiments have been adapted as overhead demonstrations. These comprise such anthological experiments as is the reaction of sodium with water (Summerlin & Ealy, 1988, p. 139), the beating mercury heart (Summerlin & Ealy, 1988,p.130; Najdoski,Mirčeski, Petruševski & Demiri, 2007), colorful demonstrations of complex formation (Bowman, 2006), simulation of X-ray diffraction experiments (Dragojlovic, 1999), rapid growth of silicate crystals (Phillips, 1988), and many more.
Methodology
The research was based on a thorough literature review of microscale experiments, and specifically, the subclass of overhead lecture experiments retrieved from the two distinguished chemistry education journals—Journal of Chemical Education and The Chemical Educator. These overhead demonstrations were compared with the more traditional macroscopic experiments to see whether the overhead approach was superior.
Only well-known experiments ('favorite chemistry demonstrations') were analyzed. These experiments included the following:
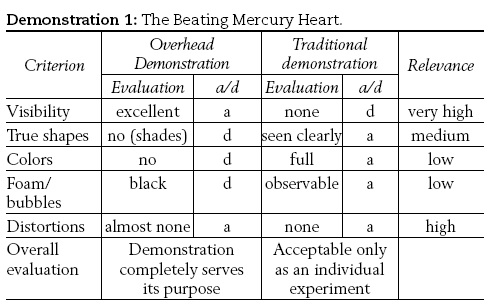
1) The beating mercury heart,
2) Reaction of sodium with water,
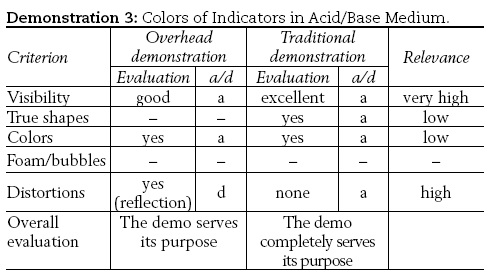
3) Colors of indicators in acidic/basic medium,
4) Growth of insoluble silicates ("silicate garden"), and
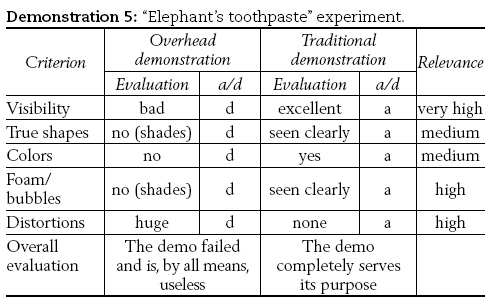
5) The "Elephant's toothpaste" demonstration
The experiments were prepared for university students in their final year (these are the students that will supposedly be high-school chemistry teachers, after their graduation).
Results
A synopsis of the microscale is given, followed by the advantages or disadvantages (a/d) for both the overhead and traditional versions that are tabulated for a given criterion, together with the relevance of that criterion. A fixed set of criteria was used for consistence, although some demonstrations did not meet the criteria. All lines of assessment reflect the opinion of the authors.
1) About 0.5 mL of mercury was just covered with diluted sulfuric acid (in a watch-glass). A nail was placed in the solution so that it barely touched the mercury. Drops of diluted hydrogen peroxide were added, which triggered oscillations of the mercury. These oscillations lasted for several minutes as the hydrogen peroxide is added.
2) A Petri dish was half-filled with distilled water. A few drops of liquid detergent (to prevent pieces of sodium from being stuck on the walls of the dish) and a few drops of phenolphthalein solution were added. Several pieces (the size of a match head) of sodium were placed one at a time in Petri dish. The sodium pieces moved across the surface and dissolved, while the solution turned purple.
3) A Petri dish containing few crystals of bromophenol blue indicator was half-filled with distilled water, and a few drops of diluted hydrochloric acid (0.1 mol/L) were added. The solution was stirred using a glass rod until it turned yellow. Then a few drops of NaOH solution with the same concentration were added, and the solution was stirred until it turned a deep bluish-purple. The results of the demonstrations are presented in Figures 1 and 2.
4) A crystal of cobalt(II) nitrate was dropped in a test-tube filled with concentrated aqueous solution of sodium (meta)silicate (various crystals of water-soluble salts of transition metals may be used together). A rapid growth of insoluble silicate(s) resulted, that can easily be followed on the screen during the "projection" of the experiment. The use of a vertical projection "Alyea-type" adaptor is vital (Phillips, 1988).
5) Aqueous solution of hydrogen peroxide with weight ratio (w) between 10% and 15% is added to aqueous solution of potassium iodide (KI) containing liquid detergent. Large amount of foam is generated. The results are shown in Figure 3.
Discussion
The results show that there are demonstrations where the microscale version of the experiment combined with the use of an overhead projector give excellent results. Thus, despite some minor shortcomings (e.g. loss of color) and some minor distortions of the direct visual perception (e.g. black bubbles and black foam appear in the projected experiment), demonstrations (1) and (2) are almost ideal as overhead demonstrations. Consequently, in a large class of students, the only practical and applicable solution to the corresponding demonstrations is to use an overhead.
It is not quite so in demonstration (3). The effect of the microscale demonstration (cf. Figure 1) is inferior to that of the macroscopic demonstration (i.e. that where beakers are used, cf. Figure 2). From an aesthetical point of view, the "loss of one dimension" (as in the projected experiment) is always less appreciative than the full 3D visual perception (as in the macroscopic experiment, using large enough beakers). Further, the information in both images presented in Figure 1 is severally distorted by the projected image of the overhead's intense light source. Of course, it is possible to use the overhead demo and things can be smoothly presented and discussed (paying attention to the distortions of the true image), but the above can better be achieved by the classical experiments with the beakers. Obviously, in such a case the "modernization" of the demonstration does not pay off. Note, by the way that the color of the indicator when the medium is basic slightly differs in transmitted (Figure 1 b) and reflected/ scattered light (Figure 2 b).
The demonstration (4) projects well, which is very important since students can follow in real time the formation/ growth of insoluble silicate crystal. However, the vertical adaptor needed for projection is not readily available. Finally, the beauty of this experiment (widely known as "silicate garden") is in the reach colors of the grown insoluble silicates, characteristic for different transition metals that could be admired only if the experiment is performed in the standard (classical) version. On the other hand, all these amazing colors are projected as highly non-attractive black shades resulting from the fact that the colored insoluble silicates are not transparent. Thus the audience is left with a highly distorted version of the reality, definitely not a good choice and one which does not serve the purpose well.
Demonstration (5) has been chosen as a typical example of an experiment that is absolutely inappropriate for projection purposes. It is historically a very important, as it is "the heart" of the first chemical oscillator (Bray, 1923). When performed in the classical way it is an astonishing demonstration with huge amount of foam being produced (cf. Figure 3 a), as a result of generation of oxygen gas bubbles. The amount and shape of the foam generated indeed resemble toothpaste squeezed off from a giant tube (hence the name "elephant's toothpaste"). In the projected demonstration, one is faced with a dark grey region "crowned" with the image of the light source (cf. Figure 3 b). Obviously, such demonstrations (or "demonstrations") where the reality is distorted to such an extent that it can not be recognized must be avoided.
Conclusions
The answer to the question posed in the introduction, seems straightforward: classical demonstrations are often irreplaceable in the course of the chemistry teaching process. It is a big delusion that it is enough for the instructor to use modern equipment (like an overhead or a projector coupled to a laptop computer) for the success of the teaching to be guaranteed. Unfortunately, it seems (at least locally, in the Republic of Macedonia) that this delusion is so widely spread that it might take time to correct it.
This problem has already been partly discussed by the famous science educator James Trefil (Trefil, 2004). The main point was that the medium (i.e. the instrumentation) can not compensate for the poor content. In his own words (regarding PowerPoint presentations):
"If you have only a few, poor quality, data points, presenting them in a beautifully colored three-dimensional graph doesn't really change anything – it's still poor quality data. Similarly, if you don't have something significant to say, having text fly in from the side of the screen doesn't really add anything significant. The problem, in both cases, is that the overpowering visual effect of the presentation can obscure the poor quality of the ideas behind it."
This, it seems to us, is a precisely located problem of why so many educators are in favor of the extensive use of modern equipment during their lectures. They might really believe it has something to do with the inevitable modernization of the educational process. While this cannot be denied in general (as there are indeed so many opportunities offered by a wise use of modern technology), it is very indicative that the modernization has actually turned into "modernization" once the teacher postpones the classes due to lack of electric power (yes, electricity breakouts still occur in Macedonia, although not very often), on a sunny day, somewhere between 10:00 am and noon. The teacher/educator can usually offer some excuse like "I wasted so much of my time to organize it as a PowerPoint presentation, that it would be a pity if students don't see it". The true answer would be, we suspect "Being dependent to the technology and heavily relying on it for a long time, I am not capable to deliver a decent lecture in the traditional way".
There are precious aids that, unfortunately, further alienate the teachers from the lecture rooms, chalk-boards and laboratory equipment: now one can order hundreds of digitalized chemistry demonstrations (Journal of Chemical Education Online, 2008) on CD's (DVD's), so she/he is free to concentrate on the real thing, and that's the education process itself. We fear that, with such a trend, it may happen that a chemistry educator might teach a class without ever performing a real chemistry experiment. And that, we dare say, will be a disaster for her/him, for the class and for the chemistry/science.
So, let's go back to the demonstration lab. There are so many wonderful things there and even more to be yet discovered.
References
Bray, W.C., A periodic reaction in homogeneous solution and its relation to catalysis, Journal of the American Chemical Society, 43, 1262–1267, 1921. [ Links ]
Bowman, D.C., A colorful look at the chelate effect, Journal of Chemical Education, 83, 1158-1160, 2006. [ Links ]
Dragojlovic, V., Using overhead projector to simulate X-ray diffraction experiments, Journal of Chemical Education, 76, 1240-1241, 1999. [ Links ]
Fowles, G., Lecture experiments in chemistry, 5th edition, Bell & Sons Ltd., London, 1959. [ Links ]
Hugerat, M., and Schwarz, P., Microscale electrolysis with disposable materials, The Chemical Educator, 13, 7-10, 2008. [ Links ]
Ibanez, J.G., Lopez-Mejia, E., and Echevarria-Eugui, J.A., Microscale environmental chemistry. Part 9: Removal of metal ions by cementation, The Chemical Educator, 12, 381-383, 2007. [ Links ]
Journal of Chemical Education Online, 2008, Chemistry comes alive. Consulted in the URL http://jchemed.chem.wisc.edu/jcesoft/cca (accessed on February 23rd, 2009). [ Links ]
Keenan, C.V., Some demonstrations with the overhead projector, Journal of Chemical Education, 35, 36-37, 1958. [ Links ]
Kolb, D., Introduction to overhead projector demonstrations (OP), Journal of Chemical Education, 64, 348, 1987. [ Links ]
Moran-Moran, M.T., and Hernandez-Esparza, M., Comparative study of the use of microscale versus macroscale techniques in the experimental determination of methanol and water solutions, The Chemical Educator, 9, 297-302, 2004. [ Links ]
Najdoski, M., Mirčeski, V., Petruševski, V. M., and Demiri, S., The mercury beating heart: modifications to the classical demonstration, Journal of Chemical Education, 84, 1292-1295, 2007. [ Links ]
Noller, C.R., Apparatus for lecture demonstration of optical activity, Journal of Chemical Education, 26, 269-270, 1949. [ Links ]
Petruševski, V.M., Monković, M., & Ivanovski, V., Laser pointers – a tool for better lecture experiments, Journal of Turkish Science Education, 3, 43-51, 2006. [ Links ]
Petruševski, V.M., Monković, M., and Šoptrajanov, B., Demonstrations as a tool for ironing-out preconceptions: 1. On the reactions of alkali metal sulfates with concentrated sulfuric acid, The Chemical Educator, 12, 71-74, 2007. [ Links ]
Petruševski, V.M., Stojanovska, M.I., and Šoptrajanov, B.T., Oscillating reactions: two analogies, The Science Education Review, 6, 68-73, 2007. [ Links ]
Phillips, D.B., A very rapidly growing silicate crystal, Journal of Chemical Education, 65, 453-454, 1988. [ Links ]
Shakhashiri, B.Z., Chemical demonstrations. A handbook for teachers of chemistry, Vol. 1, University of Wisconsin Press, Madison, 1983. [ Links ]
Shakhashiri, B.Z., Chemical demonstrations. A handbook for teachers of chemistry, Vol. 2, University of Wisconsin Press, Madison, 1985. [ Links ]
Shakhashiri, B.Z., Chemical demonstrations. A handbook for teachers of chemistry, Vol. 3, University of Wisconsin Press, Madison, 1989. [ Links ]
Shakhashiri, B.Z., Chemical demonstrations. A handbook for teachers of chemistry, Vol. 4, University of Wisconsin Press, Madison, 1992. [ Links ]
Slabaugh, W.H., The overhead projector and chemical demonstrations. Journal of Chemical Education, 28, 579-580, 1951. [ Links ]
Summerlin, L.R., and Ealy, J.I., Chemical demonstrations. A sourcebook for teachers,Vol.1,2nd edition,American Chemical Society, Washington DC, 1988. [ Links ]
Summerlin, L.R., Borgford, C.L., and Ealy, J.B., Chemical demonstrations. A sourcebook for teachers, Vol. 2, American Chemical Society, Washington DC, 1987. [ Links ]
Trefil, J., Why I never use power point when I teach: some thoughts on technology and instruction, Bulletin of the Chemists and Technologists of Macedonia, 23, 193-196, 2004. Also available at the URL http://www.mjcce.org.mk/trud.php?id=108&jd=28&start=0, (accessed February 23rd 2009). [ Links ]