Introduction
The remarkable regenerative capacity of the liver makes the basis of modern liver surgery. The description of the small-for-size syndrome by Prof. Starzl in 1975 showed, however, that the liver regenerative capacity has certain limits1. Although the size of the remnant liver was identified as the primary determinant of the development of the post-hepatectomy liver failure that characterizes the small-for-size syndrome, the accumulated experience in both extended hepatectomies and liver transplantation with reduced grafts has progressively revealed the pathogenetic relevance of portal hyperafflux and portal pressure2.
The mechanisms by which the liver regenerates after a liver injury, be it an intoxication, an infection, or a resection of liver tissue, and how it may be therapeutically modulated, have not been fully established3,4. Regenerative preconditioning, a term coined by Nagano et al.5 relies on the concept that a liver with “activated” hepatocytes tolerates a mass resection better than a liver with hepatocytes in a “quiescent” state, representing a potential approach to prevent the post-hepatectomy liver failure. Portal vein ligation (PVL) or embolization (PVE) are well-known hepatocyte stimulation techniques employed to increase the size of the future liver remnant before extensive hepatic resections, but this strategy requires approximately 4 weeks before the hepatectomy can be performed6. Both PVL and PVE have also been used experimentally just 24-48 h before liver surgery, therefore, not involving pre-operative liver growth. In experimental studies, this regenerative preconditioning strategy was associated with improved tolerance to extended liver resections5,7. Hepatic induction of Klf2 and hepatic hemodynamic effects has been involved, but the operative mechanisms of regenerative preconditioning have not been fully elucidated.
Hepatic energy metabolism and inflammation-related signaling are two relevant processes for liver regeneration following hepatic resections. Release of ATP in liver sinusoids is one of the fasted events that occur after a resection of liver mass, and purinergic signaling has been involved in triggering liver regeneration8. Most studies have also found a decrease of mitochondrial oxidative phosphorylation at the early stages of liver regeneration, followed by a recovery over normal levels at later stages for fulfilling the increased energy demands imposed by the synthesis of new cellular components9. Similarly, cytokine signaling is a consistent observation early after the resection of liver mass, and it plays major roles in the early transcriptional response of hepatocytes, in sensitizing hepatocytes to adequately respond to growth factors later in the process, and in the acute phase response10,11. Both altered energy metabolism and disarranged inflammation have been linked to the development of failed liver regeneration and post-hepatectomy hepatic failure12,13. Notably, both processes are likely to be influenced by regenerative preconditioning induced by PVL or PVE14,15.
Here, we aimed to evaluate the effects of regenerative preconditioning by PVE on hepatic energy metabolism and inflammatory mediators in liver tissue in pigs undergoing subtotal hepatectomies 24 h after a PVE.
Materials and methods
Animals and experimental design
Twenty female swine, eight Large-white, and 12 Mini-pig, (body weight: 42.0 ± 2.0 kg) were divided in two groups (n = 10/group): a) the control group, in which swine underwent a subtotal 90% hepatectomy, and b) the PVE group, in which a PVE was performed 24 h before undergoing a subtotal 90% hepatectomy.
The study was approved by the Ethics Committee for Animal Experimentation of the General University Hospital Gregorio Marañón (Madrid, Spain) and of the Centro de Cirugía de Mínima Invasión Jesús Usón (Cáceres, Spain) and conformed to the European Union Regulations on animal experiments and with the “Guide for the care and use of laboratory animals” by the American Research Council, 2011 edition (https://www.nap.edu/download/12910) and the ARRIVE guidelines.
Only female animals were included in the study because of the anatomical differences in the genitourinary system; namely, the bladder and urethra extend along the midline in male pigs, which makes more difficult to perform a wide laparotomy.
Anesthetic and surgical protocol
We performed a subtotal 90% hepatectomy under general anesthesia with fentanyl (3 μg/kg i.v.), propofol (2–-4 mg/kg i.v.), and atracurium (0.6 mg/kg i.v.), following previously described protocols16. After the resection of liver mass, all animals were maintained anesthetized for 24 h until the end of the experiments. After 24 h of observation, a new laparotomy was performed to collect the tissue samples, and then the animals were euthanized with deep anesthesia and an intravenous bolus of potassium chloride. The measurement of liver function by the indocyanine green retention test and the collection of blood and tissue samples were performed at the beginning of the surgery before deresection of liver mass (Bas), and 15 min (T15m) and 24 h (T24h) after performing the subtotal 90% hepatectomy in conditions of hemodynamic stability. A more detailed description of the present experimental model can be found in a recent publication16.
Regenerative preconditioning by PVE
Based on the concept of regenerative preconditioning introduced by Nagano et al., we performed a PVE to stimulate hepatocyte proliferation in the future liver remnant. The PVE was performed by interventional radiology 24 h before undergoing the subtotal 90% hepatectomy. The embolization occluded 90% of the total liver mass, corresponding to the parenchyma that would later be resected, as previously described7.
Histological evaluation
Histological sections prepared from formalin-fixed paraffin-embedded liver tissue samples were stained with hematoxylin-eosin, and evaluated blindly by two pathologists (IP, ES). A score of histological damage was elaborated from seven parameters: congestion, hemorrhage, periportal edema, septal edema, endothelial detachment, necrosis, and apoptosis. Each parameter was scored: no damage (0), low (1), moderate (2), and severe (3). To give more weight to the parameters reflecting more acute damage, the final Histological Damage Score was calculated as S(congestion + 2*hemorrhage + periportal edema + septal edema + 2*endothelial detachment + 2*necrosis + 2*apoptosis). Therefore, the score ranged from 0 to 33 points, with a score ≥ 3 being considered histological damage.
Measurement of the protein expression of cytokines in liver tissue
We studied the expression of the following molecular mediators of inflammation: TNF-α, MCP-1, IL-1β IL-10, IL-4, IL-5, IL-6, and IL-8 following standard Western-blotting protocols using poly-acrylamide gel electrophoresis and nitrocellulose membranes (Bio-Rad, Mississauga, Ontario). Proteins were extracted after homogenizing and extracting flash-frozen liver tissue samples (50-60 mg) in buffer (100 mmol/L NaCl, 10 mmol/L TRIS-Cl (pH 7,6) 1 mmol/L EDTA (pH 8), and 1 mg/ml aprotinin, 100mg/ml PMSF). We used the following primary antibodies at 1:1.000 dilution: polyclonal rabbit anti-(rat) IL-1β (cat# 500-P80), IL-10 (cat# 500-P139), and TNF-α (cat# 0204M073RB) primary antibodies from Peprotech, monoclonal rabbit anti-(mouse) IL-6 (cat#12912) from cell signaling, polyclonal rabbit anti-MCP1 (cat# ab7814) from Abcam, and polyclonal rabbit anti-IL-4 (cat# 35227), anti-IL-5 (cat# 41062), and anti-IL-8 (cat# 41063) from Signalway Antibody LLC (College Park, MD, USA). Membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibodies (1:2000 dilution), and protein expression was detected using the ECL system (Amersham, Oakville, Ontario). Relative protein expression was determined using a scanner (BioRad GS 800) and the Quantity ONE 1.0 Analysis Software, versión 4.5.2.
Measurement of the mRNA expression of cytokines in liver tissue
The mRNA expression in the remnant liver tissue of the molecular mediators of inflammation TNF-α, MCP-1, IL-1β, and IL-10 was evaluated in a 7500 Fast Real-time PCR System (Applied Biosystems™) using commercially available reagents and primers validated for porcine genes from AnyGenes (Paris, France), following the manufacturer's instructions.
Concentration of adenine nucleotides in liver tissue
The concentration of ATP, the adenosine diphosphate (ADP), and the AMP was measured in flash-frozen tissue samples, which were lyophilized and stored at –80°C until biochemical determination.
ATP was measured by a spectrophotometric method based on the coupling of the ATP requiring 3-phosphoglycerate phosphorylation with an oxidation-reduction system (1,3-diphosphoglycerate/glyceraldehyde-3-P) involving a NAD+-linked enzyme. ATP was determined by measuring the decrease in absorbance at 340 nm. AMP was enzymatically converted to ADP, and ADP to ATP, by means of myokinase and pyruvate kinase, respectively.
The ratio ATP: ADP and the energy load were also calculated. For the energy load, the formula used was: (ATP + (ADP/2))/(ATP + ADP + AMP). Its value ranges from 0 (all AMP) to 1 (all ATP).
Statistical analysis
The statistical analysis was performed using the SPSS version 23.0 for Mac (IBM Corporation, Armonk, NY), and the graphs were created using GraphPad Prism for Windows v8.4.3 (GraphPad Software, LLC). The results were expressed as mean ± standard error of the mean. For the analysis of differences between groups, the mixed error-component model was used. With this model, we also looked for differences between groups in the 3-time points: Bas, T15m, and T24h. A p < 0.05 with a confidence level of 95% was considered statistically significant.
Results
General characteristics and outcomes of hepatic surgery
All PVE and the 90% hepatectomies were successfully completed. All animals were hemodynamically stable at the end of the surgery. Mortality after the 90% hepatectomy was similar in both groups (Control: 2 of 10 (20%) vs. PVE: 3 of 10 (30 %), p = 1.0). In the control group, one animal died in the immediate posterative time, and the other one died more than 12 h after surgery due to a cardiac embolism. Mortality in the PVE group occurred in three animals, all of them more than 12 h after the surgery.
Neither the PVE nor the subtotal hepatectomy induced major changes in circulating liver biochemistry markers (Table 1), except for a small increase of AST before the operation in the PVE group compared with the control group (p = 0.047). The circulating concentrations of ALT, AST, AP, and bilirubin tended to increase after the operation, but the changes were not statistically significant compared with baseline, and all of them were similar in the control and the PVE groups. Similarly, the INR tended to worsen at 24 h after the operation in both groups (p = 0.299).
Table 1 Evolution of blood laboratory parameters, liver function, and histological tissue damage in pigs of the control and the PVE groups undergoing a 90% hepatectomy
| Group | Time point | p | |||
|---|---|---|---|---|---|
| Bas | T15m | T24h | |||
| ALT (IU/l) | Control | 51.1 ± 8.8 | 37.7 ± 3.8 | 79.9 ± 15.9 | 0.445 |
| PVE | 40.7 ± 5.2 | 43.8 ± 3.9 | 48.8 ± 5.0 | ||
| p | 0.566 | 0.493 | 0.360 | ||
| AST (IU/l) | Control | 60.8 ± 7.9 | 119.3 ± 24.2 | 617.9 ± 170.5 | 0.131 |
| PVE | 95.7 ± 13.9 | 177.5 ± 30.3 | 342.8 ± 65.4 | ||
| p | 0.047 | 0.092 | 0.161 | ||
| AP (IU/l) | Control | 267 ± 56 | 255 ± 57 | 779 ± 167 | 0.149 |
| PVE | 299 ± 45 | 267 ± 31 | 670 ± 94 | ||
| p | 0.728 | 0.207 | 0.202 | ||
| Bilirubin (mg/dl) | Control | 0.39 ± 0.10 | 0.51 ± 0.09 | 1.72 ± 0.21 | 0.564 |
| PVE | 0.25 ± 0.03 | 0.40 ± 0.06 | 1.35 ± 0.14 | ||
| p | 0.881 | 0.464 | 0.969 | ||
| INR | Control | 0.90 ± 0.03 | 0.91 ± 0.03 | 1.94 ± 0.12 | 0.299 |
| PVE | 0.94 ± 0.05 | 0.90 ± 0.06 | 1.45 ± 0.10 | ||
| p | 0.439 | 0.605 | 0.30 | ||
| Histological damage score | Control | 0.78 ± 0.36 | 5.89 ± 0.84 | 4.11 ± 0.87 | 0.123 |
| PVE | 1.40 ± 0.54 | 4.50 ± 0.50 | 3.00 ± 0.84 | ||
| p | 0.696 | 0.022 | 0.347 | ||
| R15 | Control | 17.5 ± 3.4 | 52.1 ± 4.9 | 58.6 ± 4.1 | 0.028 |
| PVE | 13.7 ± 3.1 | 43.8 ± 12.2 | 61.9 ± 8.4 | ||
| p | 0.621 | 0.049 | 0.086 | ||
Values shown are mean ± SEM.
ALT: alanine amino transferase; AP: alkaline phosphatase; AST: aspartate amino transferase; Bas: baseline; INR: international normalized ratio; PVE: portal vein embolization;
R15: retention time 15 of indocyanine green; T15m: 15 min post-hepatectomy; T24h: 24 h post-hepatectomy
As in our prior study7, the 90% hepatectomy resulted in worsening of the histological damage score (Table 1), and the PVE had a slightly protective effect at the T15m time point (Control: 5.89 ± 0.84 vs. PVE: 4.50 ± 0.50, p = 0.022). Although the 90% hepatectomy worsened the indocyanine green R15 in both groups (p = 0.028, Table 1), liver function was slightly better in the PVE group compared with the control group at the T15m time-point (Control: 52.1 ± 4.9 vs. PVE: 43.8 ± 12.2, p = 0.049).
Changes in energy metabolism in liver tissue
The 90% hepatectomy had a drastic impact on hepatic energy metabolism, as assessed by the concentration of ATP, ADP, and AMP in the remnant liver tissue (Table 2). At baseline, the concentration of energy metabolites in liver tissue was similar in the control group and the PVE group, except for a small increase of AMP in the later (p = 0.009) The changes of energy metabolites became evident in both groups immediately after the resection of liver mass (T15m) and persisted up to the 24 h time point. They consisted of a decrease of ATP (p = 0.027) associated with concomitant increases of ADP (p = 0.003) and AMP (p < 0.001). Of note, the concentration of ATP was higher and the concentrations of ADP and AMP were lower in pigs of the PVE group compared with the control group at both the T15m and T24h time-points. Consequently, both groups presented important decreases of the ATP/ADP ratio and of the energy load immediately after the operation, but these calculated parameters were lower in pigs of the control group compared with pigs that underwent a PVE before the hepatic surgery (p < 0.001) (Fig. 1).
Table 2 Concentration of ATP, ADP and AMP in liver tissue from pigs of the Control and the PVE groups undergoing a 90% hepatectomy
| Group | Time point | p | |||||
|---|---|---|---|---|---|---|---|
| Bas | p (Bas-T15m) | T15m | p (Bas-T24h) | H24 | |||
| ATP (μmol/mg tissue) | Control | 4.06 ± 0.21 | < 0.001 | 2.17 ± 0.05 | < 0.001 | 1.18 ± 0.06 | 0.027 |
| PVE | 3.90 ± 0.17 | 2.78 ± 0.06 | 2.09 ± 0.03 | ||||
| p | 0.573 | <0.001 | <0.001 | ||||
| ADP (μmol/mg tissue) | Control | 1.32 ± 0.07 | < 0.001 | 2.81 ± 0.06 | < 0.001 | 3.24 ± 0.08 | 0.003 |
| PVE | 1.37 ± 0.02 | 2.41 ± 0.01 | 2.87 ± 0.23 | ||||
| p | <0.001 | 0.082 | 0.499 | ||||
| AMP (μmol/mg tissue) | Control | 0.85 ± 0.04 | < 0.001 | 1.26 ± 0.04 | < 0.001 | 1.90 ± 0.03 | < 0.001 |
| PVE | 0.99 ± 0.02 | 1.04 ± 0.03 | 1.43 ± 0.12 | ||||
| p | 0.009 | 0.001 | 0.001 | ||||
Values shown are mean ± SEM. A mixed-model analysis was used to assess statistically significant differences between groups and time points.
Bas: baseline; PVE: portal vein embolization; T15m: 15 minutes post-hepatectomy; T24h: 24 hour post-hepatectomy.
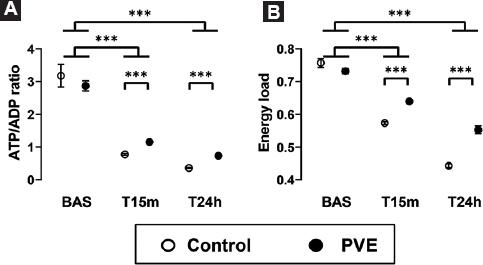
Figure 1 Changes in ATP/ADP. A: ratio. B: energy load. After a 90% hepatectomy in normal pigs (Control group) and in pigs that underwent a PVE 24 h before the hepatic surgery (PVE group). The liver tissue samples were obtained just before the resection of liver mass (Bas), and at 15 min (T15m) and 24 h (T24h) later. The box and whiskers graphs show the mean ± the standard error of the mean. ***p < 0.001.
Changes in inflammatory mediators in liver tissue
There were no changes of inflammatory mediators associated with the performance of the PVE, as assessed by the mRNA and protein expression of TNF-α, IL-1β, MCP-1, IL-10, IL-4, IL-5, IL-6, and IL-8 in liver tissue in the control and the PVE groups at baseline (Figs. 2 and 3, Table 3).
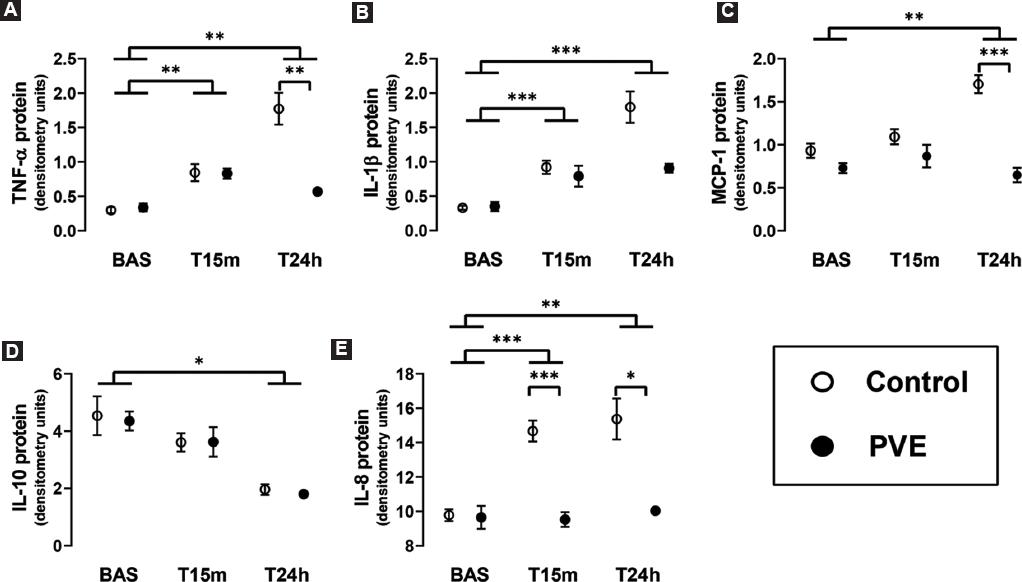
Figure 2 Densitometry analyses of Western-blots evaluating the expression. A: TNF-α. B: IL-1β. C: MCP-1. D: IL-10. E: IL-8 (E) proteins in the remnant liver tissue after a 90% hepatectomy in normal pigs (Control group) and in pigs that underwent a PVE 24 h before the hepatic surgery (PVE group). The liver tissue samples were obtained just before the resection of liver mass (Bas), and at 15 min (T15m) and 24 h (T24h) later. The box and whiskers graphs show the mean ± the standard error of the mean. *p < 0.05, **p < 0.01, ***p < 0.001.
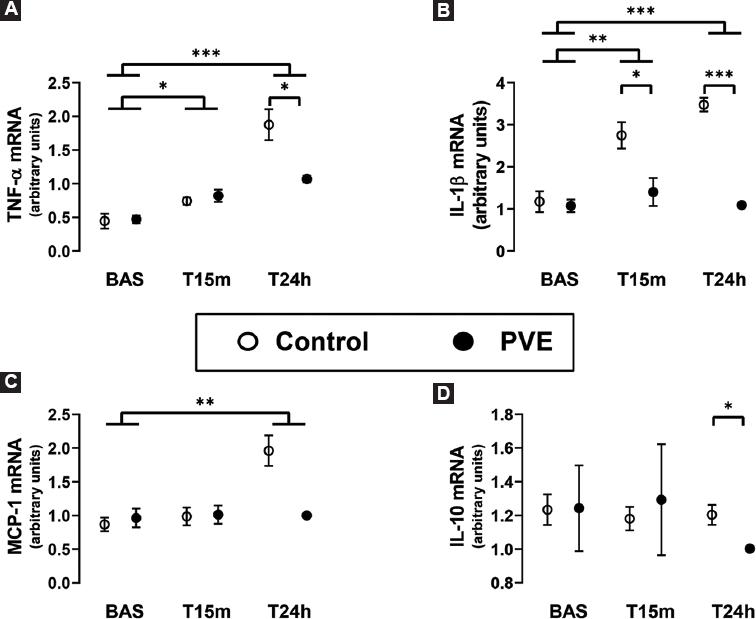
Figure 3 Real-time RT-PCR evaluation of the hepatic mRNA expression. A: TNF-α. B: IL-1β. C: MCP-1. D: IL-10. In the remnant liver tissue after a 90% hepatectomy in normal pigs (Control group) and in pigs that underwent a PVE 24 h before the hepatic surgery (PVE group). The liver tissue samples were obtained just before the resection of liver mass (Bas), and at 15 min (T15m) and 24 h (T24h) later. The box and whiskers graphs show the mean ± the standard error of the mean. *p < 0.05, **p < 0.01, ***p < 0.001.
Table 3 Expression of pro-inflammatory and anti-inflammatory cytokines in liver tissue from pigs of the control and the PVE groups undergoing a 90% hepatectomy, as assessed by Western blotting
| Group | Time point | P | |||||
|---|---|---|---|---|---|---|---|
| Bas | p (Bas-T15m) | T15m | p (Bas-T24h) | H24 | |||
| TNF-α (densitometry units) | Control | 0.299 ± 0.043 | 0.002 | 0.845 ± 0.123 | 0.002 | 1.772 ± 0.232 | 0.012 |
| PVE | 0.338 ± 0.058 | 0.831 ± 0.074 | 0.569 ± 0.036 | ||||
| p | 0.593 | 0.919 | 0.006 | ||||
| IL-1β (densitometry units) | Control | 0.330 ± 0.030 | < 0.001 | 0.922 ± 0.956 | < 0.001 | 1.797 ± 0.228 | 0.131 |
| PVE | 0.350 ± 0.068 | 0.791 ± 0.152 | 0.907 ± 0.063 | ||||
| p | 0.794 | 0.480 | 0.119 | ||||
| MCP-1 (densitometry units) | Control | 0.931 ± 0.082 | 0.564 | 1.093 ± 0.087 | 0.002 | 1.705 ± 0.104 | 0.002 |
| PVE | 0.729 ± 0.058 | 0.868 ± 0.131 | 0.648 ± 0.084 | ||||
| p | 0.067 | 0.180 | <0.001 | ||||
| IL-10 (densitometry units) | Control | 4.538 ± 0.680 | 0.396 | 3.609 ± 0.320 | 0.034 | 1.962 ± 0.188 | 0.919 |
| PVE | 4.353 ± 0.331 | 3.623 ± 0.514 | 1.802 ± 0.008 | ||||
| p | 0.811 | 0.981 | 0.344 | ||||
| IL-4 (densitometry units) | Control | 1.152 ± 0.312 | 0.234 | 0.917 ± 0.204 | 0.142 | 0.656 ± 0.135 | 0.816 |
| PVE | 1.289 ± 0.227 | 0.924 ± 0.104 | 0.701 ± 0.103 | ||||
| p | 0.727 | 0.976 | 0.746 | ||||
| IL-5 (densitometry units) | Control | 1.474 ± 0.123 | 0.120 | 1.009 ± 0.134 | 0.268 | 1.215 ± 0.104 | 0.916 |
| PVE | 1.573 ± 0.252 | 1.141 ± 0.110 | 1.256 ± 0.007 | ||||
| p | 0.730 | 0.462 | 0.881 | ||||
| IL-6 (densitometry units) | Control | 1.340 ± 0.100 | 0.094 | 1.812 ± 0.091 | 0.266 | 1.732 ± 0.284 | 0.886 |
| PVE | 1.211 ± 0.142 | 1.428 ± 0.402 | 1.298 ± 0.159 | ||||
| p | 0.473 | 0.369 | 0.800 | ||||
| IL-8 (densitometry units) | Control | 9.786 ± 0.339 | < 0.001 | 14.671 ± 0.612 | 0.006 | 15.372 ± 1.193 | < 0.001 |
| PVE | 9.657 ± 0.666 | 9.530 ± 0.430 | 10.034 ± 0.027 | ||||
| p | 0.866 | <0.001 | 0.022 | ||||
Values shown are mean ± SEM of densitometry analyses of the corresponding Western blots. A mixed-model analysis was used to assess statistically significant differences between groups and time points.
Bas: baseline; PVE: portal vein embolization; T15m: 15 min post-hepatectomy; T24h: 24 h post-hepatectomy.
The resection of liver mass induced the hepatic expression of TNF-α, IL-1β, and IL-8 proteins immediately after the hepatectomy (T15m), and they remained elevated 24 h later (Fig. 2 and Table 3). The protein expression of MCP-1 and IL-10 was unchanged at T15m, but MCP-1 increased and IL-10 decreased at 24 h after the hepatectomy. Importantly, the changes of TNF-ba, MCP-1, and IL-8 were significantly attenuated or completely prevented in the group of pigs undergoing PVE before the 90% hepatectomy, with the effect on IL-8 being noted already at the early T15m time-point. The hepatic expression of IL-4, IL-5, and IL-6, however, was not affected either by the PVE nor by the resection of liver mass.
In addition to protein expression, we also determined the impact of PVE and 90% hepatectomy on the mRNA expression of TNF-α, IL-1β, MCP-1, and IL-10 (Fig. 3). The genetic expression of these inflammatory mediators was mostly in accordance with the findings of their protein expression. Furthermore, the real-time RT-PCR analyses of liver tissue revealed significantly lower expressions of IL-1βeta mRNA at T15m and T24h and of IL-10 mRNA at T24h in the PVE group compared with the control group, which had not been detected at the protein level.
Discussion
The previous studies have reported beneficial hemodynamic, analytical, and histological effects of regenerative preconditioning induced by the occlusion of portal vein in animals undergoing extended resections of liver mass5,7, but its effects on molecular pathways relevant for liver regeneration still need to be elucidated. In the present study, we evaluated the changes on hepatic energy metabolism and inflammatory mediators that resulted from regenerative preconditioning induced by PVE performed 24 h before a subtotal 90% hepatectomy in pigs. We found that regenerative preconditioning by PVE attenuated the deterioration of hepatic energy metabolism and the elevation of pro-inflammatory cytokines in the remnant liver as early as 15 min after the resection of liver mass, which was associated with slight improvements of liver pathology and liver function as assessed by liver histology and the indocyanine green retention test, respectively.
A number of studies have reported considerable changes of the concentration of adenine nucleotides and other energy compounds after a resection of liver mass. Such changes differ depending on the time interval from the surgical operation as well as on the extent of the hepatic resection. After the resection of liver mass, an early, almost immediate, drastic decrease of the hepatic concentration of total adenine nucleotides, not necessarily associated with energy consumption or mitochondrial impairment, has been mainly related with the release of ATP by hepatocytes and non-parenchymal cells, and it appears to constitute a relevant trigger for liver regeneration8,9,17. Increased energy consumption or bioenergetic impairment at this stage, manifested as decreases of ATP with parallel increases of ADP and AMP or as mitochondrial dysfunction, however, has been related with extended hepatic resections or other conditions associated with impaired hepatocyte proliferation or impending liver failure12,15,18,19. As in prior studies and consistent with the development of post-hepatectomy liver failure, pigs undergoing 90% hepatectomies presented an immediate decrease of ATP associated with concomitant increases of ADP and AMP, resulting in decreases of the energy load and of the ATP/ADP ratio that persisted up to the 24-h time point. Although it did not prevent them, regenerative preconditioning by PVE significantly attenuated these changes, supporting the improvement of mitochondrial function observed in prior studies in rats with a similar approach14.
The impact on the expression of inflammatory mediators in the liver tissue remnant was a second major effect of regenerative preconditioning by PVE noted in the present study. Although cytokine activation and signaling plays a pivotal role in the normal recovery of liver mass, an excessive or aberrant production of inflammatory mediators leads to liver damage and is detrimental for liver regeneration, as it has been observed in many pathologic processes13,20-23. Here, the resection of liver mass led to an induction of pro-inflammatory (TNF-α, IL-1β, MCP-1, and IL-8) and a decrease of anti-inflammatory (IL-10) proteins. The fact that most of these changes were observed at both the mRNA and the protein level, and that other mediators (IL-4, IL-5, and IL-6) remained unchanged, provided consistency to our observations. Regenerative preconditioning by PVE was associated with a partial attenuation or even complete prevention of some of those changes. The prevention of the induction of IL-1β mRNA and of IL-8 protein was observed almost immediately after the resection of liver mass, suggesting a potential mechanism by which regenerative preconditioning may diminish the development of post-hepatectomy liver failure. The mechanistic relevance of other effects of regenerative preconditioning that were observed at 24 h, for example, the attenuation of the increases of TNF-α, MCP-1, and IL-8, is less straightforward. A survival bias is unlikely to explain the difference between the control and the PVE groups because mortality was similar in both groups, but the changes observed still may be the consequence rather than the cause of decreased liver damage. Nonetheless, attenuation of the expression of these mediators has been shown to decrease liver damage in other studies22-24.
Conclusion
In conclusion, performance of subtotal hepatectomies leading to the small-for-flow syndrome in pigs was characterized by severe hepatic energy impairment and by the induction of a pro-inflammatory state in the remnant liver. Regenerative preconditioning by PVE improved energy metabolism and attenuated the expression of pro-inflammatory cytokines in the remnant liver tissue, suggesting potential hepatoprotective mechanisms of this strategy.











 nueva página del texto (beta)
nueva página del texto (beta)


