1. INTRODUCTION.
Corrosion of reinforcing steel in concrete structures exposed to environments contaminated with chlorides is the most common cause of premature deterioration (Hansson, 1984; Pech-Canul and Castro, 2002). The above leads to large economic losses as well as the reduction of the useful life of the structures (Valipour et. al., 2014). In recent decades, numerous investigations have been carried out in this field to analyze the causes and characteristics of this phenomenon and provide solutions to this important pathology of concrete. Normally a good quality concrete keeps the steel protected due to its high alkalinity, in addition the concrete cover acts as a physical barrier that prevents the access of aggressive agents from the surrounding environment (Hansson, 1984). These properties are lost at an early age, so the use of natural materials or additives that improve the properties of concrete and extend its useful life is a very important aspect to consider.
Currently the additives form an integral part of the components in cement-based mixtures (Ramírez-Arellanes et. al., 2012). However, despite the effectiveness of synthetic additives to improve the different properties of concrete, these are highly polluting. In that sense, the investigation of natural additives from plants and their use in concrete are becoming more and more relevant.
The Opuntia genus belongs to the Cactaceae family and is also known as cactus pear plant (Sáenz et. al., 2004). One of the main uses of the Cactaceae family is directly related to the production of mucilage. The stems and leaves secrete a viscous liquid, which is a gum or hydrocolloid, mainly composed of polysaccharides. Polysaccharides are composed of long chains of monosaccharide units, resulting in polymeric carbohydrate molecules (Zhang et.al., 2019). This complex carbohydrate has potential uses as an additive for several industrial products (Sáenz et. al., 2004). It has been used as a water purifier, as an additive in lime mortars to improve its adhesion, as well as an additive capable of modifying the properties in mortars both in the fresh and hardened state (León-Martínez et. al., 2010). Its use in concrete varies according to the properties to be modified, such as: workability, aspects such as paste homogeneity, as well as the setting time of the mixture (Zhang et. al., 2019). Also, it is considered a potential source of industrial hydrocolloids with many application in the food industry (Cárdenas et. al., 1997; Sáenz et. al., 2004; León-Martínez et. al., 2010).
Opuntia ficus-indica, is a native plant of Mexico that grows in arid and semi-arid areas. Currently its cultivation for commercial reasons has spread to countries such as Italy, the United States, Chile and Argentina (Torres-Acosta, 2007; Martinez-Molina et. al., 2015). In Mexico, this plant is called Nopal and is a great source of food for the general population, as well as for livestock. Since ancient times, the gel produced by this cactus has been used to paint and cover adobe walls, as well as for the maintenance and preservation of churches and historic buildings in Latin America (Chandra et. al., 1998; Torres-Acosta and Martínez-Madrid, 2005; Torres-Acosta, 2007).
Different studies agree that the compounds present in the Nopal mucilage are very varied, being able to find proteins, as well as different types and compositions of polysaccharides (Chandra et. al., 1998). In general, the carbohydrate composition in the mucilage contains varying proportions of l-arabinose, d-galactose, l-rhamnose and d-xylose as the main sugar units (León-Martínez et. al., 2011). Some natural polymers are capable of modifying specific properties of cementitious materials during construction (Peschard et al., 2004). Some properties of cement mortars in the fresh state can be improved with the addition of water-soluble polymers. The cement mixtures modified with these polymers have a high water retention than ordinary mortars. This behavior is mainly due to the hydrophilic parts of the polymers fixing the water molecules in the fresh mixture, preventing drying by evaporation and absorption in the surrounding porous material (Knapen and Van Gemert, 2009).
Ramírez-Arellanes et. al. (Ramírez-Arellanes et. al., 2012) analyzed the effect of Nopal mucilage in cement paste; determining that the setting times increased with the addition of this natural additive. Also, they reported that there were changes in microscopy of mixtures with mucilage. Other authors report that the sizes of calcium hydroxide crystals are reduced (Chandra et. al., 1998) and in the presence of water soluble polymers the microstructure of the concrete is modified (Peschard et. al., 2004; Knapen and Van Gemert, 2009).
Other preliminary findings suggest that small concentrations of Nopal gel could be useful as a corrosion inhibitor for reinforcing steel in chloride-contaminated mortar. There was an improvement in the durability of the Nopal gel specimens, due to an increase in polarization resistance and a decrease in corrosion-induced cracking (Martinez-Molina et. al., 2015).
This present research focuses on the study of Nopal mucilage as a modifying additive for the electrochemical properties of reinforced concrete. In that regard, the objective of this investigation is to provide a solution that minimizes the damages caused by the corrosion of the reinforcing steel, this being the pathology that most affects the reinforced concrete structures. An important parameter of the analysis is to determine the corrosion rate of the reinforcing steel with the addition of different concentrations of Nopal mucilage and analyze its effect over time.
2. EXPERIMENTAL PROCEDURE.
2.1 Nopal mucilage extraction.
The cactus leaves that were used had a fresh state of preservation and were free of spines. To proceed with the extraction of the Nopal mucilage, the following procedure was carried out: i) the cleaning of the leaves was carried out to eliminate traces of dust and other residues, ii) the leaves were cut into pieces of 1cm x 1cm to extract the gel as much as possible and iii) the pieces were mixed with water to obtain three concentrations of mucilage in a Nopal-water weight ratio of 1: 1, 1: 2 and 1: 3, seen on Figure 1.

Figure 1 a) Fresh cactus leaves, b) Prickly pear cactus mixed with water y c) Filtration process of Nopal mucilage.
The extraction of the Nopal mucilage was carried out by two methods, which are described below. Maceration at room temperature, in which each Nopal mixture with water was allowed to macerate for 48 hours for later use in the concrete. After this time the solution began to acquire a darker tone and a certain smell of decomposition, as other authors affirm (Chandra et. al., 1998). The following extraction method was maceration applying temperature in this case the Nopal and water mixtures were placed on a grill applying a temperature of 95 degrees Celsius for 10 minutes. Then it was allowed to standstill for 24 hours, at which time the solution was incorporated into the concrete. In both extraction methods before incorporating the Nopal mucilage into the concrete the solution was filtered.
2.2 Design of concrete mixes.
The concrete mixes were designed using a CPC30R cement (Type II ASTM-C-150) taking into account a characteristic strength of 250 kg/cm2. The water/cement ratio used was 0.45 for each of the mixtures made. River sand was used as a fine aggregate and the coarse aggregate from crushed stone had a maximum size of 20 mm. The reinforcing steel formed by 3/8 inch grade 42 and the corrugated bars had no surface treatment. The proportions for concrete mixtures are shown in Table 1.
Table 1 Concrete mixture ratio for each cylindrical sample (CS) and prismatic sample (PS).
| Materials | Amount of materials per sample | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO | CO+1-1N | CO+1-2N | CO+1-3N | CO+1-1NT | CO+1-2NT | CO+1-3NT | ||||||||
| CS | PS | CS | PS | CS | PS | CS | PS | CS | PS | CS | PS | CS | PS | |
| Cement (kg) | 0.041 | 0.231 | 0.041 | 0.231 | 0.041 | 0.231 | 0.041 | 0.231 | 0.041 | 0.231 | 0.041 | 0.231 | 0.041 | 0.231 |
| Sand (kg) | 0.081 | 0.452 | 0.081 | 0.452 | 0.081 | 0.452 | 0.081 | 0.452 | 0.081 | 0.452 | 0.081 | 0.452 | 0.081 | 0.452 |
| Gravel (kg) | 0.127 | 0.711 | 0.127 | 0.711 | 0.127 | 0.711 | 0.127 | 0.711 | 0.127 | 0.711 | 0.127 | 0.711 | 0.127 | 0.711 |
| Water (l) | 0.019 | 0.105 | - | - | - | - | - | - | - | - | - | - | - | |
| Nopal mucilage (l) | - | - | 0.019 | 0.105 | 0.019 | 0.105 | 0.019 | 0.105 | 0.019 | 0.105 | 0.019 | 0.105 | 0.019 | 0.105 |
The specimens were designed with a 30 mm of concrete cover between the edge of the bars and the sides of the cube. Therefore, the specimens were 7 cm wide, 10 cm long and 10 cm high, and the exposed area of the steel bars in contact with the concrete was 18 cm2, as seen in Figure 2. Each steel bar was coated with adhesive tape on the mortar-air interface as described by other authors (González et. al., 2004; Caré and Raharinaivo, 2007; Poursaee, 2010).
The preparation of the mixtures was carried out at room temperature inside the laboratory. Once all the solid elements were mixed, the Nopal mucilage was added according to the concentration obtained. Only water was added to the control sample, in the rest of the specimens the water was replaced by the Nopal mucilage. After 24 hours of fabrication, the samples were placed in water for 28 days, time of which the curing of concrete was carried out. After that, they remained partially submerged for the rest of the test period in a 3% sodium chloride solution, simulating a marine environment. The distance between the upper edge of the samples and the solution was maintained around 2 cm.
The first electrochemical tests were started after 24 hours of mixing the materials and for the next 270 days. All tests were performed keeping the specimens in the curing solution at the beginning and then in the sodium chloride solution. The techniques used to analyze the electrochemical behavior of reinforcing steel were the following: Open Circuit Potential, Electrochemical Noise and Linear Polarization Resistance.
Table 2 shows the nomenclature used to identify each sample with the different concentrations of Nopal mucilage, as well as the control sample.
Table 2 Identification of the samples.
| Samples | Nopal-water weight ratio |
Extraction method | Nomenclature |
|---|---|---|---|
| 1 | ---- | No Nopal mucilage (CO) | CO |
| 2 | 1:1 | Maceration at room temperature (N) | CO+1-1N |
| 3 | 1:2 | Maceration at room temperature (N) | CO+1-2N |
| 4 | 1:3 | Maceration at room temperature (N) | CO+1-3N |
| 5 | 1:1 | Maceration applying temperature (NT) | CO+1-1NT |
| 6 | 1:2 | Maceration applying temperature (NT) | CO+1-2NT |
| 7 | 1:3 | Maceration applying temperature (NT) | CO+1-3NT |
2.3 Compression resistance technique.
The Compression Resistance technique is one of the most widely used tools in the analysis of the mechanical properties of concrete. Three concrete samples were designed for each concentration of Nopal mucilage, including samples without mucilage. The compression resistance test was performed 28 days after the curing process of all specimens, while remaining wet. Cylindrical specimens were designed from PVC pipes with a height / diameter ratio equal to 2, with the following dimensions: 4.3 cm of diameter and 8.6 cm of height. The specimens were scaled, taking into account that in each step of this project it was ensured to guarantee the lowest consumption of energy and materials. The parameters were defined according to ASTM C39 (Dúran-Herrera et. al., 2012; Rahmani et. al., 2013).
2.4 Parameters of electrochemical techniques.
2.4.1 Open circuit potential technique.
The Open Circuit Potential technique is one of the most widely used tools for the analysis of reinforced concrete structures (Morozov et. al., 2013). The measurement of Open Circuit Potential was carried out against a Saturated Calomel Electrode (SCE). In this case, a measurement of the steel electrodes of each specimen was made. The final value obtained was the average of the three measurements. The first reading was taken 24 hours after the samples were made, and weekly measurements were made for a period of 270 days. For this, a multimeter was used connecting one terminal to the working electrode and the other to the reference electrode of Calomel. To measure the Open Circuit Potential, the reference electrode was placed inside the curing solution and the saline solution, as close as possible to the working electrodes. It was taken into account that the tip of the calomel reference electrode was separated from the bottom of the solution container. Table 3 shows the ranges of corrosion potential values for reinforced concrete structures and the corrosion probability criteria according to ASTM C876 (Morris et. al., 2002; Pérez-Quiroz et. al., 2008).
2.4.2 Electrochemical noise techniques.
Corrosion processes such as: generalized and localized corrosion, stress corrosion cracking, as well as passivation phenomena, generate spontaneous fluctuations in the electrode's free corrosion potential (Gusmano et. al., 1997). An ACM Instruments Auto ZRA potentiostat was used to analyze the electrochemical noise of all samples. The readings for each test were 1024 data with a sampling rate of one data per second. In addition, the standard method of analysis of three nominally identical electrodes was used (Cottis, 2001).
One of the most important advantages offered by this electrochemical technique is that its application does not imply any artificial alteration of the system during the test time (Legat et. al., 2004). A parameter widely used in the analysis of the electrochemical noise signal is noise resistance (Rn), defined as the ratio of standard deviations of potential and current noise, according to equation (Bing et. al., 2007):
where, σv is the standard deviation of potential noise and σi is the standard deviation of current noise. A linear trend removal of the time series of potential and current was performed.
Some authors have analyzed the relationship between noise resistance (Rn) and polarization resistance (Rp), concluding that they can be considered equivalent for many systems (Aballe et. al., 2001; Girija et. al., 2007).
2.4.3 Linear polarization resistance technique.
The Linear Polarization Resistance technique is a very versatile tool frequently used for the electrochemical studies of reinforcement steel embedded in concrete (Andrade et. al., 2001). One of its main advantages is that it allows to determine the kinetics of the corrosive process. According to other studies, a voltage signal was applied in the range of ± 20 mV over the corrosion potential (Ecorr), a current signal being recorded as a response (Poursaee, 2010). For the measurement of the Linear Polarization Resistance a sweep rate of 10 mV/min was applied. In addition, the reference electrode and graphite counter electrode were placed inside the curing solution and saline solution. Both electrodes were placed next to each other and as close as possible to the working electrodes. The polarization resistance can be determined through the expression (2), established as the slope of the polarization curve around the corrosion potential, Ecorr (Andrade and Alonso, 1996; Morris et al., 2002):
where Rp is the polarization resistance (Ω), ΔI is change in current (A) and ΔE is change in potential (V) (Poursaee, 2010). According to the equation (3) proposed by Stern-Geary, it is possible to determine the corrosion rate of the reinforcing steel through a constant of proportionality B. This equation states that the current density Icorr is inversely proportional to the Rp (Hansson, 1984; Morris et. al., 2002):
The ranges of corrosion rate values in terms of the useful life of reinforcing steel in concrete are shown in Table 4 (Andrade and Alonso, 1996).
Table 4 Intervals of corrosion rate related to the degree of corrosion of the steel in the concrete in terms of useful life.
| Corrosion current
Icorr(μA/cm2) |
CR
(mm/y) |
Condition of the rebar |
|---|---|---|
| Icorr< 0.1 | <0.001 | Negligible. |
| Icorr 0.1 - 0.5 | 0.001-0.005 | Low to moderate corrosion. |
| Icorr 0.5 - 1.0 | 0.005-0.010 | Moderate to high corrosion. |
| Icorr> 1.0 | > 0.010 | High corrosion rate. |
From the Icorr values, the efficiency of the Nopal mucilage as a corrosion inhibitor of the reinforcing steel in concrete was determined, according to the following equation (Díaz-Cardenas et al., 2017):
where: I.E. is the efficiency of the inhibitor, Icorr is the corrosion current density (μA/cm2) without inhibitor and I´corr is the corrosion current density (μA/cm2) with inhibitor.
3. RESULTS AND DISCUSSION.
3.1 Compression strength.
Table 5 shows the average values of compressive strength after 28 days of concrete curing.
Table 5 Average values of compressive strength after 28 days.
| Samples | Compressive strength (kg/cm2) |
|---|---|
| CO | 248.9 |
| CO+1-1N | 223.5 |
| CO+1-2N | 234.9 |
| CO+1-3N | 246.5 |
| CO+1-1NT | 225.8 |
| CO+1-2NT | 234.6 |
| CO+1-3NT | 244.1 |
As can be seen, there are no significant differences in compression resistance values, regardless of the method of extracting the Nopal mucilage. On the other hand, as can be seen after 28 days, all samples with Nopal mucilage maintain compression resistance values lower than the control sample. This is due to the fact that the Nopal mucilage traps water and that decreases the hydration rate of the cement at early ages, due to the hydrophilic part of the polymers present in the mucilage fix the water molecules in the fresh mixture (Knapen and Van Gemert, 2009). For samples with a concentration 1-3 of Nopal mucilage, there was a decrease in resistance between 2.4 and 4.8 kg/cm2, however, for concentration 1-1 ratio of mucilage, the decrease in compression resistance remained between 23.3 and 25.4 kg/cm2 respect to the control samples. Some authors describe a similar trend and show that the presence of polysaccharides in the mucilage solution are the main causes of this behavior (Chandra et. al., 1998). It is also known that Nopal mucilage as a natural additive in concrete is able to delay cement setting (Peschard et. al., 2004). However, according to Chandra et. al. (Chandra et. al., 1998) in the long term, the cactus mucilage favors the increase in compression resistance, exceeding the control sample values.
3.2 Open circuit potential.
The corrosion potential values for all samples with Nopal mucilage are detailed in Figure 3.
During the concrete curing process, it is clear that the potential of all samples acquires very noble values, between -90 and -50 mV. These values remain in the range of a 10% probability of corrosion (Pérez-Quiroz et. al., 2008). The high alkalinity, as well as the presence of moisture and oxygen in the concrete pore network, are factors that influence these potential values. Under these conditions, the steel develops an oxides passive layer of compact and warterproof (Hansson, 1984).
All samples with Nopal mucilage, have a drop in their values and reach the range of intermediate corrosion risk. Possibly, due to the presence of chloride ions on the surface of the steel, localized corrosion occurs and consequently the breakdown of the passive layer (Caré and Raharinaivo, 2007). Although with the advance of the exposure period, the ideal conditions of the concrete seem to be maintained and the potential values are gradually recovered, approaching more noble potential values, between -210 and -60 mV. Unlike the control sample that reaches values close to -500 mV with a probability of corrosion of 90%. The CO+1-3N sample showed the best behavior with very noble values of potential, around 60 mV at 270 days of testing.
Various factors influence the behavior of the Nopal mucilage specimens. Cactus gel acts as a cementing retarding additive (Zhang et. al., 2019). In addition, it is able to retain moisture for a longer period of time, because the polysaccharides have a water retention character and decrease the speed of drying of the concrete (Chandra et. al., 1998). Therefore, the process of micro-cracking of concrete is reduced, a phenomenon that takes place especially in hot climates (Zhang et. al., 2019).
3.3 Electrochemical noise.
As an example, the following time series shows the current fluctuations of the samples with Nopal mucilage obtained by maceration at room temperature, see Figure 4 and Figure 5.

Figure 4 Time series of current at 28 days of concrete curing for the following samples: a) CO, b) CO+1-1N, c) CO+1-2N and d) CO+1-3N.
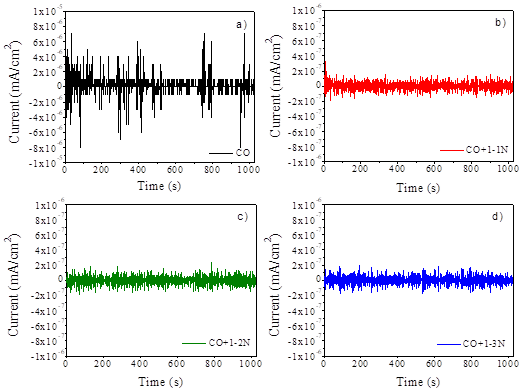
Figure 5 Time series of current at 161 days of concrete curing for the following samples: a) CO, b) CO+1-1N, c) CO+1-2N and d) CO+1-3N.
After 28 days of concrete curing, all current time series show a similar behavior with fluctuations that reach values of up to 2x10-7 mA/cm2. These low current values are indicative of a passivation state in the reinforcing steel. The passive layer of steel evolves over time (Hansson, 1984) and through this technique these small changes in current values can be detected (Gusmano et al., 1997; Cottis, 2001). Only CO+1-3N sample has some transients with values of up to 8x10-7 mA/cm2.
With the advance of the exposure time to the aggressive environment, a significant change in the behavior of the time series for the CO sample can be seen. In general, a change in the values of current time series of up to an order of magnitude is observed, with some transients reaching values of up to 8x10-6 mA/cm2. This behavior can be associated to the presence of chloride ions on the surface of the steel, which cause the rupture of the oxide layer (Hansson, 1984). On the other hand, the samples with Nopal mucilage for day 161 show a decrease in the current values, and no abrupt changes are observed. This is an indication that the steel passivation steel is maintained as well as the conditions for it to remain in that state.
Figure 6 shows the noise resistance values determined from the standard deviation values of voltage and current of the analyzed time series.
At the beginning of the tests, a progressive increase in Rn is observed. These results show that the cactus mucilage does not adversely affect the curing process of concrete and guarantees the conditions for the reinforcing steel to develop a passive film. After 150 days of testing, the control sample shows a fall in Rn values close to 2x104 Ω*cm2, with significant fluctuations in their values. This behavior indicates that the onset of corrosion consists of a series of severe localized events (Legat et. al., 2004).
At the end of the test period all samples with Nopal, keep values greater than 1x105 Ω*cm2, with the exception of the CO+1-3NT sample, which maintained a slightly higher performance respect to the control sample. In contrast, the CO+1-3N sample reached values greater than 4x105 Ω*cm2 significantly improving the electrochemical properties of reinforced concrete. This response evidences part of the advantages offered by this Nopal gel, as it not only acts as a retarder of setting concrete, but also as an additive that can improve the electrochemical response of reinforcing steel, delay the onset and active spread of corrosion in reinforcing steel (Martinez-Molina et. al., 2015).
3.4 Linear Polarization Resistance.
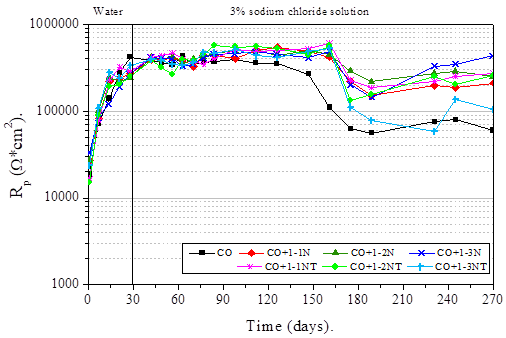
In Figure 7 it can be observe the Rp values obtained from linear polarization resistance technique.
In general, it can be seen that these results show a similar trend to noise resistance values. The good performance of the Nopal mucilage within the concrete matrix is evident, because despite presenting a drop in Rp values after 150 days of exposure to the aggressive environment, progressively the resistance of all samples increases at the end of the trial period.
A very important property that influences the behavior described by the samples with Nopal mucilage is its high viscosity, a parameter that improves the workability of the mixture as well as the homogeneity of the concrete (Knapen and Van Gemert, 2009; León-Martínez et. al., 2014). Some studies affirm that certain natural polymers (polysaccharides) present in the Nopal mucilage, react with the cement compounds forming complexes that reduce the porosity in concrete, mainly because they are smaller compounds (Chandra et. al., 1998; Ramírez-Arellanes et. al., 2012).
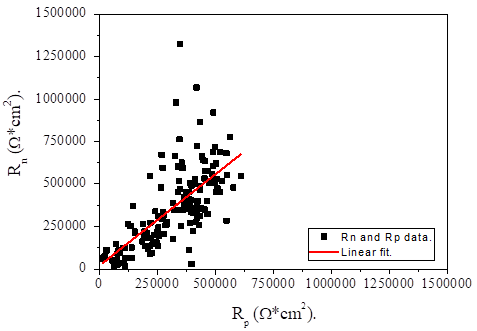
A linear correlation was made from the Rn and Rp results of all samples, as shown in Figure 8.
From this analysis a correlation coefficient with a value of 0.695 was obtained. This value indicates a reasonable correlation between the results of both electrochemical techniques (Kearns et al., 1996), taking into account that a value of cero indicates that there is no correlation and a value of one indicates a very good correlation. This result confirming that both techniques are equivalent and suitable for the electrochemical study of reinforcing steel in concrete. In fact, many studies of the electrochemical behavior of reinforcing steel in particular with the use of these techniques have been reported in the literature (Andrade et. al., 2004; Legat et. al., 2004; Bing et. al., 2007; Poursaee, 2010).
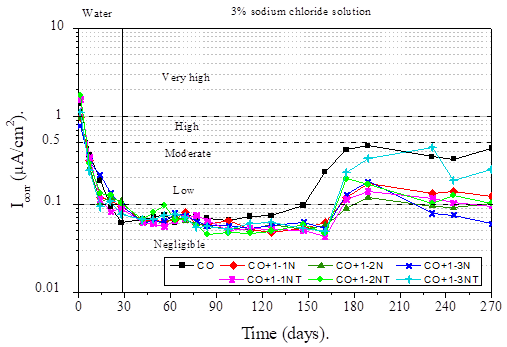
The corrosion rate is inversely proportional to the Linear Polarization Resistance values from which the Icorr was determined for all samples (Andrade and Buják, 2013). According to some researches the proportionality factor (constant B) varies from 13 to 52 mV. In this study on the analysis of the corrosion rate of the reinforcing steel in concrete, a value of B = 26 mV was applied (Andrade et. al., 2004). All corrosion rate values in terms of current density are shown in Figure 8.
During the curing process of concrete, a rapid decrease in Icorr values can be observed until values below 0.1 μA/cm2 are reached in the negligible corrosion speed range (Andrade and Alonso, 1996). At these values, steel possibly already developed a state of passivity due to the presence of oxygen, moisture and a highly alkaline medium (Hansson, 1984). With the advance of the exposure time to the aggressive medium all samples with mucilage maintain very low Icorr values. This behavior, as stated by other studies, is possibly due to the fact that this natural additive is capable of reducing the diffusion coefficient of chlorides, caused by an increase in the viscosity of the solution of the concrete pores (Ramírez-Arellanes et. al., 2012).
At the end of the test period, samples with Nopal mucilage maintained corrosion rate values between 0.1 and 0.2 μA/cm2, except for the CO+1-3N sample, which maintained values lower than 0.08 μA/cm2 in the range of negligible corrosion rate (Andrade and Alonso, 1996) These indicated that the Nopal mucilage not only improves the electrochemical properties of steel, but also that in the presence of chlorides it can keep the reinforcing steel protected for a longer time (Martinez-Molina et. al., 2015). On the contrary, the control sample is maintained in the range of moderate corrosion rate.
Table 6 shows the values of corrosion current density and Nopal mucilage efficiencies reached at the end of the test period. As can be seen, the highest efficiency of Nopal mucilage inhibitor was 86% for the CO+1-3N sample, with a 1-3 concentration of Nopal mucilage extracted by maceration at room temperature for 48 hours.
4. CONCLUSIONS.
This research work was aimed at the study of the electrochemical properties of reinforcing steel in concrete with the addition of Nopal mucilage. An analysis was made from three concentrations of Nopal mucilage taken up by two extraction methods. The favorable effect of this natural additive was appreciated by restricting the onset of corrosion and protecting the reinforcing steel.
The conclusions are as follows:
For the samples with the Nopal mucilage concentration 1-3, the highest compression resistance values were achieved, taking into account that this natural additive acts as a retardant of the setting of the concrete.
From the open circuit potential, the favorable effect of the Nopal mucilage was appreciated as an additive that can delay the corrosion of reinforcing steel in concrete. The CO+1-3N sample reached very noble potential values at the end of the trial period, being the most favorable dosage.
During the concrete curing process, all samples exhibited a similar behavior and a rapid increase in the noise resistance (Rn) and the polarization resistance (Rp) values were observed. It can be affirmed that the Nopal mucilage within the concrete matrix maintains the ideal conditions for the steel to acquire a state of passivation.
All samples with Nopal mucilage showed the highest Rn and Rp values with respect to the control sample for a longer period of time. A reasonable correlation coefficient was obtained between both electrochemical results from Rn y Rp, with a value of 0.695.
The Nopal mucilage was able to delay the onset of corrosion in concrete and maintain a corrosion rate between negligible and low until the end of the test period.
The mixture that presented the best electrochemical behavior was CO+1-3N, with an efficiency of 86% for the concentration of Nopal mucilage of 1:3, obtained by maceration at 48 hours without cooking at 95o Celsius.











 nueva página del texto (beta)
nueva página del texto (beta)