The Asteraceae family is one of the most diverse among the vascular plants. It is estimated to comprise about 10 % of angiosperms worldwide (Pyšek 1997, Funk et al. 2005). Many species of this family are heliophilous (Suárez-Mota et al. 2018) and have a high production of seeds with a specialized structure (pappus) that facilitates their dispersion (Yuan & Wen 2016). The seeds of some Asteraceae species may have a dormancy, which allows the formation of seed banks (Fenner & Thompson 2005). These seed traits facilitate the colonization of disturbed environments by Asteraceae species (Diniz et al. 2010), which have a high invasive capability, to the extent of being considered weeds (Villaseñor et al. 2011, Yuan & Wen 2016). Despite this, weeds may have important contributions to ecosystems due the fact that harbor beneficial insects such as pollinators and pest controllers, or even due to improvement of soil nutrients (Blaix et al. 2018). This functional group offers also cultural services, such as medicinal, insecticidal, herbicidal, alimentary, or ornamental uses that some species of Asteraceae have (Heinrich et al. 1998, Stepp & Moerman 2001, Ghani et al. 2011, Guzmán-Pantoja et al. 2012, Nenaah et al. 2015, García-Herrera et al. 2014, Cicevan et al. 2016, Benvenuti et al. 2017, Prebeg et al. 2019).
There are life traits related to seed biology (viability, longevity, dormancy and storage temperature) that should be considered for weed management and/or implementation of production protocols for potentially useful plant species (Rodríguez-Arévalo et al. 2017). Loss of viability in seeds is related to an increase of reactive oxygen species (i.e., singlet oxygen, hydrogen peroxide and hydroxyl radical) causing DNA damage, protein adducts and lipid peroxidation (Sano et al. 2016, Kurek et al. 2019). On the other hand, seed longevity is defined as the period that the seeds remain alive (Kochanek et al. 2010, Sano et al. 2016, Buitink & Leprince 2018). Under storage conditions of low humidity (< 10 %) and temperature (< 5 oC or subzero) the cytoplasm becomes glassy, which impedes the mobility of the molecules avoiding chemical reactions implicated in seed aging (Buitink et al. 2000). As temperature increases, viscosity of cytoplasm is recovered and chemical reactions occur due to the availability of water. Therefore, at high temperatures seed aging accelerates, reducing viability and delaying seed germination (Sano et al. 2016). The optimal storage temperature for the maintenance of seed viability varies among plant species; for example, seeds of the Asteraceae Helianthus annus L. stored for four months under three temperatures (4-5, 21-22 and 35 oC) showed the lowest germination at the highest temperature. Furthermore, seed viability diminished with aging (Ghasemnezhad & Honermeier 2007). Similar pattern is observed in the weeds Bacharis dracundifolia DC., Senecio vulgaris L., Solidago altissima L., S. nemoralis Aiton and S. shorti Torr. & A. Gray which seeds reduced germination with aging and at room temperatures of 25-27 oC (Walck et al. 1997, Gomes & Fernándes 2002, Ndihokubwayo et al. 2016). Knowledge of seed traits allows us to determine appropriate storage conditions, since, if seeds are viable and long-lived but do not germinate, they may have dormancy, which could make them susceptible to prolonged storage.
The Mexican species Aldama dentata La Llave, Stevia origanoides Kunth, Roldana barba-johannis (DC.) H. Rob. & Brettell (Senecio barba-johannis) and Verbesina virgata Cav. (Asteraceae) are ruderal species, distributed in central Mexico with recognized ethnobotanical utility. Aldama dentata has been used for the alleviation of gastritis (Chena-Becerra et al. 2014) and it is an important element of flora for beekeeping (Cadena-Rodríguez et al. 2019), S. origanoides has antioxidant properties (Medina-Medrano et al. 2018), meanwhile R. barba-johannis is used as bioinsecticide (Céspedes et al. 2004), organic fertilizer, firewood and for crafting (Aguilar-Santelises & del Castillo 2015). Also, molecules with antimicrobial activity (eudesmane triols) have been isolated from species of Verbesina (Martínez et al. 1983, Mora et al. 2013). Given the potential utility of these species, the objective of this study was to determine the viability and longevity (i.e., percentage and timing of germination) in seeds of A. dentata, S. origanoides, R. barba-johannis and V. virgata stored under two different temperature conditions (5 °C and room temperature). We hypothesize that the seeds will have enough longevity to form seed banks, which will make them potential candidates to ex situ storage. Therefore, we expect the following: a) seeds will decrease their viability and germination capacity (percentage and timing of germination) as they age, and b) their longevity will be greater when stored at the lower temperature (5 °C).
Materials and methods
Study area, species and seed collection. Seeds of R. barba-johannis, S. origanoides and V. virgata were collected along the roads that connect the localities of San Juan Tlacotenco and Coajomulco, in the National Park “El Tepozteco”, in Tepoztlán, Morelos, Mexico (18° 53' 20" to 19° 05' 30” N; 99° 02' 00” to 99° 12' 55" W, 2,300 m asl). The mean annual temperature at the study area is 15.9 °C and mean annual precipitation is 1,477.9 mm (Cruz-Fernández et al. 2011). The predominant vegetation types are crassicaule xerophytic scrub and monospecific or mixed Quercus forests dominated by Quercus rugosa Née (Cruz-Fernández et al. 2011). Seeds of Aldama dentata were collected at the “Chamilpa” campus of the Universidad Autónoma del Estado de Morelos in Cuernavaca, Morelos (18º 59´ 00´´ N and 99º 14´ 13´´ W, 1,909 m asl). The campus is located in the north of the urban area of Cuernavaca. Remnants of the original vegetation show that this was pine-oak forest and pine forest; however, due to urbanization, most of the vegetation is secondary and ruderal vegetation (Solalinde-Vargas 2014).
During November and December 2015, capitula (heads) of each species (30 individuals/species) were collected. This collection was conducted when the achenes (uniseminal, dry and indehiscent fruit, hereafter referred to as seeds) separated easily from the capitulum. Once detached, the seeds were quantified, excluding any visibly non-vigorous seeds (soft consistency and grayish color). Visibly healthy seeds (hard, dark and without signs of herbivory) were randomly assigned to the storage conditions described in the following section.
Storage temperature effect on seed longevity (seed germination and mean germination time). Seed longevity of the four species of Asteraceae was evaluated monthly by measuring both the percentage of germination and the mean germination time. For each species, and prior to carrying out the germination experiments, 176 batches of 50 healthy and vigorous seeds were placed randomly in aluminum foil envelopes; 88 of these envelopes were stored in a cold room (constant temperature 5 ± 0.5 °C) at the Laboratorio de Botánica Estructural in the Centro de Investigación en Biotecnología (CEIB) and the other 88 envelopes of each species were stored at room temperature in CEIB (16.45 ± 1.94 °C, Figure 1) since they were collected. The seeds from eight envelopes stored at room temperature and at 5 °C were germinated (400 seeds for each temperature treatment). Due to the seeds of these species germinate at the end of summer, the first germination trial was carried out in July 2016 using seeds that were 222 days of age, and the subsequent experiments were performed with seeds of 253, 284, 314, 345, 375, 406, 437, 465, 496 and 526 days of age. The final experiment was conducted in May 2017.

Figure. 1 Mean monthly temperature under room conditions for storage of the seeds of four Asteraceae species. Temperature was measured daily from January 2016 until May 2017. Data were obtained from UAEM weather station (Comisión Nacional del Agua). Points and dispersion lines represent mean values and 1SE, respectively
Before each monthly germination experiment, the seeds were disinfected with sodium hypochlorite (1 %, cloralex®) for 30 seconds under constant agitation, after disinfection the seeds were rinsed three times with sterile distilled water in order to remove traces of chlorine. Finally, the 50 seeds of each envelope were placed in Petri dishes (90 mm in diameter × 15 mm in height) lined with filter paper (Whatman No. 2) and moistened with 4 mL of distilled water. To prevent moisture loss, the Petri dishes were sealed with Parafilm (Pechiney-Plastic packaging, model PM-996, USA) and Clingfilm (Kirkland Signature, model 26761, USA). Thus, 16 Petri dishes per species were considered each month, half of these had seeds that were stored at 5 oC and the rest had seeds stored at room temperature. Petri dishes were placed for 18 days in a germination chamber (Scorpion Scientific, Bioclimatic Environmental Chamber A-50624, Mexico) equipped with white warm fluorescent light, with a photoperiod of 12 h light-12h darkness and a constant temperature of 25 °C. A seed was considered to have germinated when the radicle emerged. Each experiment was carried out for 12 days and the number of germinated seeds was recorded daily.
Mean germination time was calculated according to Ranal & García de Santana (2006) as:
Where ti represents the day of the record of germination data (from 1 to 18), ni is the number of germinated seeds on day i and k is the number of days of germination recording (k = 18 days). This formula weighs the seed germination according to the time at which they germinated. Thus, if most of the seeds germinate during the initial days, the value of the mean germination time will be low (fast germination) and will increase as a greater number of seeds germinate in the final days of the experiment.
Seed viability. Along with the germination experiments, the viability of the seed lots of each species was evaluated monthly using 1 % tetrazolium (TTZ, 2,3,5-triphenyltetrazolium chloride) (Moreno 1984). TTZ is employed to evaluate seed viability based on the respiratory activity of the tissues, in which the TTZ reaction consists of the reduction of TTZ to the red triphenyl-formazan. If the seed is alive (viable), then there will be activity of the enzyme dehydrogenase, which will reduce the compound (Moreno 1984). To determine the percentage of viability of each species, four samples of 80 seeds were stored in aluminum foil envelopes at room temperature for the same period as that described before (4 envelopes × 4 species × 11 months). Once the TTZ test was carried out, the seeds of each species were observed under a stereoscope microscope (Leica, model EZ4). If the embryo of the seeds became red, the seeds were considered viable. In contrast, seeds of white-pink coloration and those that were partially dyed or with absence of coloration were considered non-viable. Finally, the number of viable seeds was determined, and the viability percentage was calculated for each species.
Statistical Analyses. To determine whether seed germination of each species varied in response to the two storage temperatures and their age, a generalized linear model (GLM) with binomial error and logit link function was performed (Crawley 1993, 2013). In this analysis, the factors were storage temperature (5 °C and room temperature) and species (A. dentata, R. barba-johannis, V. virgata and S. origanoides), the covariable was seed age, the dependent variable was the occurrence of germination. To determine whether the mean germination time of the four species varied depending on seed age and storage temperature, a covariance analysis was performed for normal data (Crawley 1993, 2013). In this analysis, the covariable was seed age, the factors were identity of the species and temperature and the dependent variable was mean germination time. Finally, the effect of seed longevity on viability was determined using a GLM with binomial error and logit link function, where the factor was identity of the species, the covariable was seed age and the dependent variable was the viability or otherwise of the seed. Contrasts tests between the coefficients of the models were applied where statistical differences were detected (Crawley 1993, Bretz et al. 2011). All statistical analyses were conducted using R version 3.4.3. (R Core Team 2017), with the multcomp package (Hothorn et al. 2008).
Results
Storage temperature effect on seed longevity (seed germination and mean germination time). The identity of the species and seed age had a statistically significant effect on seed germination (Table 1). The highest germination values corresponded to A. dentata and S. origanoides, while V. virgata and R. barba-johannis had the lowest germination values (Contrast test, P < 0.05, Table 2). At the end of the experiment, the germination of seeds stored at 5 °C was (hereafter, we report
Table 1 Effect of the Asteraceae species, storage temperature, seed age and their interactions on seed germination of A. dentata, S. origanoides, R. barba-johannis and V. virgata seeds. Degrees of freedom (D.F.), χ2 and P-value of Generalized Linear Model with binomial error and logit link function are shown.
| Source of variation | D.F. | χ2 | P |
|---|---|---|---|
| Species | 3 | 107.07 | < 0.00001 |
| Temperature | 1 | 0.15 | 0.69 |
| Seed age | 1 | 996.72 | < 0.00001 |
| Species × Temperature | 3 | 26.58 | 0.0367 |
| Species × Seed age | 3 | 146.43 | < 0.00001 |
| Temperature × Seed age | 1 | 0.14 | 0.70 |
| Species × Temperature× Seed age | 3 | 22.67 | < 0.00001 |
Table 2 Mean ± 1SE of germination, mean germination time (MGT) and viability of seeds of four ruderal Asteraceae species. Different letters show statistically differences among species (Contrast test, P < 0.05), letters should be read within each column.
| Species | Germination (%) | MGT (days) | Viability (%) |
|---|---|---|---|
| Aldama dentata | 29.81 ± 1.06ª | 1.44 ± 0.77b | 59.54 ± 12.92ª |
| Roldana barba-johannis | 27.75 ± 0.73ª | 1.07 ± 0.75c | 54.20 ± 11.46ab |
| Stevia origanoides | 15.88 ± 0.77b | 1.63 ± 0.77a | 47.5 ± 10.59ab |
| Verbesina virgata | 16.42 ± 0.79b | 0.87 ± 0.58b | 38.29 ± 10.49b |

Figure. 2 Proportion of germinated seeds of four ruderal Asteraceae species in response to seed age (time since collection) and without considering seed storage temperatures. Points and dispersion lines represent mean values and 1SE, respectively
All of the interaction terms in the model were significant, except temperature × seed age (Table 1). Considering the species × storage temperature interaction, the highest germination was presented by A. dentata when its seeds were stored at 5 °C (Table 3), followed by the seeds of this species stored at room temperature and by S. origanoides, the seeds of which had also high seed germination at both temperatures (Table 2). Both R. barba-johannis and V. virgata presented the lowest germination under both storage conditions (Table 3). Regarding the triple interaction between species × temperature × seed age, germination decreased with seed aging (Figure 3a-h). It was observed that, at room temperature, A. dentata and V. virgata have continuous patterns of decreased germination over time (Figure 3a-b), while this decrease in germination is less pronounced in S. origanoides and in R. barba-johannis (Figure 3c-d).
Table 3 Mean ± 1SE values of proportion of germinated seeds in four ruderal Asteraceae species, in which seeds were stored under two temperature conditions (room and 5°C). Different letters denote statistically significant differences (Contrast test P < 0.05)
| Species | Room | 5 °C |
|---|---|---|
| (16.45 ± 12.92 °C) | ||
| Aldama dentata | 0.28 ± 0.01b | 0.31 ± 0.01a |
| Roldana barba-johannis | 0.15 ± 0.01c | 0.16 ± 0.01c |
| Stevia origanoides | 0.27 ± 0.01b | 0.27 ± 0.01b |
| Verbesina virgata | 0.15 ± 0.01c | 0.17 ± 0.01c |
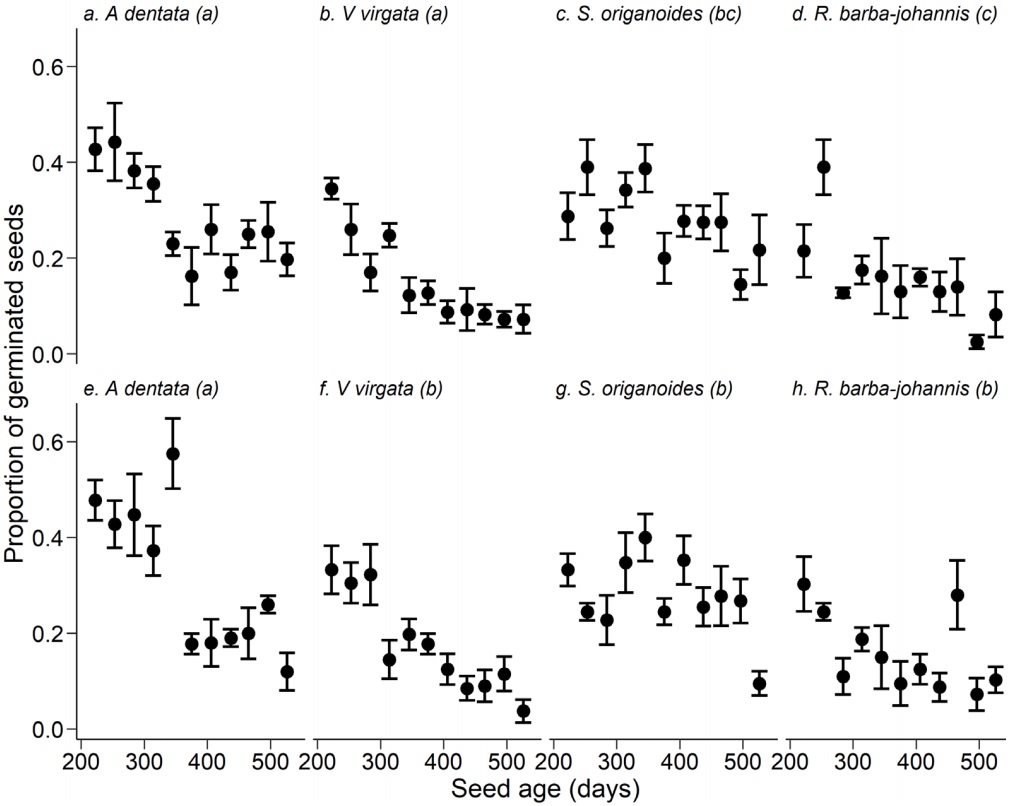
Figure. 3 Proportion of germinated seeds throughout seed aging in four Asteraceae species when stored at room (a-d) and 5 °C (e-h) temperatures. Points and dispersion lines represent mean values and 1SE, respectively. Different lowercase letters in parenthesis indicate statistically significant differences (Contrast test P < 0.05)
As with germination percentage, mean germination time was not influenced by storage temperature (χ2 = 2.76, df = 1, P = 0.09). However, there were statistically significant differences among species (χ2 = 137.60, df = 3, P < 0.0001), seeds of V. virgata had the fastest germination, followed by R. barba-johannis. Stevia origanoides had the slowest seed germination, meanwhile A. dentata was the second species with slow germination (Table 2, Contrasts test, P < 0.05).
Seed age also influenced the mean germination time (χ2 = 281.22, df = 1, P < 0.0001); this value was higher when seeds were 222-250 days old (from the time of collection) and mean germination time decreased as the seeds got older (Figure 4a-h). The interaction of species × temperature was statistically significant (χ2 = 10.03, df = 1, P < 0.05). At both seed storage temperatures, S. origanoides and A. dentata (5 oC) had the highest values of mean germination time (Figure 4a, c, e, g), while the lowest values corresponded to V. virgata (Figure 4b, f). In R. barba-johannis, mean germination time alternated between high and low values when stored at room temperature (Figure 4d), and germination occurred in less time when stored at 5 °C (Figure 4d).
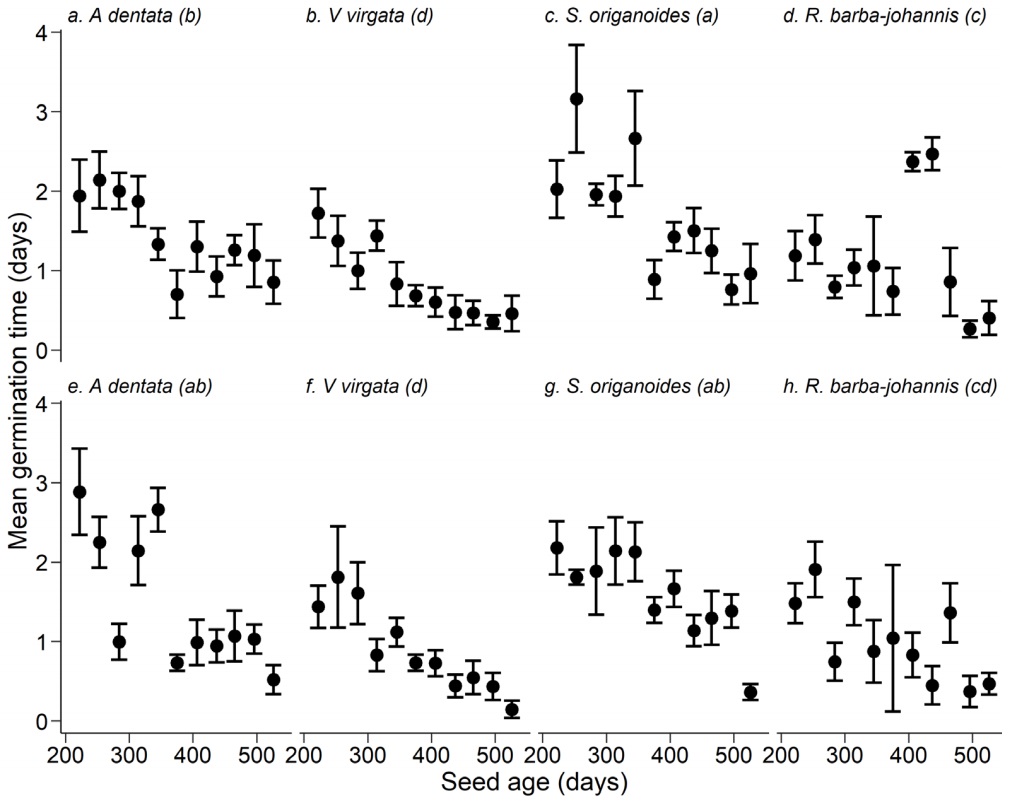
Figure. 4 Mean germination time along seed aging of four Asteraceae species when they were stored at room (a-d) and 5 °C (e-h) temperatures. Points and dispersion lines represent mean values and 1SE, respectively. Different lower-case letters in parentheses indicate statistically significant differences (Contrast test P < 0.05)
Seed viability. The percentage of seed viability diminished as the seeds aged (χ = 44.79, df = 1, P < 0.001). Among species, the statistical differences (χ = 70.21, df = 3, P < 0.0001) are based on the fact that A. dentata seeds had the highest viability, while the lowest seed viability corresponded to V. virgata. Roldana barba-johannis and S. origanoides had similar and intermediate seed viabilities. The interaction between species × seed age did not affect mean germination time (χ = 6.15, df = 3, P = 0.10). This means that, in the evaluated time, the decline in seed viability was equal among the species as they aged.
Discussion
Anthropogenic disturbance of natural ecosystems favors the presence of weeds and ruderals (Sakai et al. 2001), as is the case of many Asteraceae species (Van Etten et al. 2017). This functional group has highly ecological (i.e., maintenance of insect biodiversity, input nutrients into soil) and ethnobotanical values (food, traditional medicine, ornamental). Therefore, is important to determine optimal seed storage conditions that will maximize their viability and longevity. This study shows that seed viability and germination percentage of the studied Asteraceae species decreased with seed natural ageing, and that seed storage temperature had no effect on these parameters.
Storage temperature effect on seed germination and mean germination time. Storage temperature affects seed physiological processes (Visscher et al. 2016). For example, low seed storage temperature could prolong seed longevity if seeds have low moisture content (Probert et al. 2009). There is evidence that cool temperatures act to increase seed lifespan in orthodox (desiccation-tolerant) seeds (Ellis 1991); according to the data base of the Kew Royal Botanical Gardens (https://data.kew.org/sid/) seeds of A. dentata, S. origanoides and V. virgata, and also seeds of the genus Roldana have orthodox seeds, thus is probable that low temperatures prolong their seed longevity. Seed germination is regulated by the levels of abscisic (ABA) and gibberellic (GA) acids, through the expression of genes involved in ABA and GA biosynthesis. NCDE genes are related to ABA and are up-regulated when seeds are exposed at high temperatures (i.e., 34 °C); unlike, over expression of GA 20-oxydase genes occurs at lower temperatures (22 °C), as found in Arabidopsis thaliana (Toh et al. 2008). This is complemented with the mobility or kinetic model which establish that high temperatures accelerate biochemical reactions in seeds provoking seed aging (Buitink et al. 2000, Ballesteros & Pence 2017) and this is manifested in a loss of viability and delayed germination (Sano et al. 2016). It was expected that seed longevity in the plant species studied would be prolonged at 5 °C, but neither seed longevity nor seed germination were affected by these seed storage temperature conditions. This lack of effect of temperature on seed longevity has been reported previously (Dickie et al. 1990), where variation in seed storage temperature, particularly at sub-zero temperatures, did not affect seed longevity in Lactuca sativa L. (Asteraceae). It is possible that a prevalent room temperature (of around 18 °C) during storage may not be high enough to increase seed germination and, consequently, seed longevity. Previous studies have shown that temperature did not affect germination in orthodox seeds of the Asteraceae Helianthus annuus L., when seeds were stored at 5 °C and 20 °C (Brunick 2007); however, germination increased when these seeds were stored at 25 °C (Rodríguez et al. 2018). It is possible that the storage temperatures used in this study did not include the limits that would have caused an effect on seed germination. For future research, we recommend assessing the effect of colder temperatures on seed germination, such as that used to preserve Bidens seeds (-20 °C, Xuan et al. 2016). There are other factors not considered here that can affect seed germination, such as light requirements which may vary in plant species. Some Taraxacum species respond differently when their seeds germinate under different light conditions; moreover, depending on the species; the interaction of light and temperature affect germination (Luo & Cardina 2012). For future research, the inclusion of light preferences for germination could evidence different percentages of germination for the studied species.
According to Sano et al. (2016), one of the main symptoms of seed aging is delayed germination. However, in this study, the opposite was observed in the four species of Asteraceae: germination of seeds sowed in the first experiment was slower than in older seeds. In ecological terms, it has been hypothesized that delayed germination may act to avoid competition between seeds (Tielbörger & Prasse 2009). The results of this study indicate that, although young seeds delay their mean germination time (non-deep physiological dormancy, Baskin & Baskin 2004), they also present a higher percentage of germination compared to old seeds, which could be a strategy to avoid intra-specific competition. It is also possible that, given to the typically stressful environmental inhabited by ruderal species (i.e., high temperatures and water scarcity), species of these environments could delay their germination until favorable conditions occur (Tielbörger & Prasse 2009). Timing of seed germination is related with the permanence of species in variable environments, those genotypes with a high variation on this trait could adapt better to seasonal uncertainty, especially if the germination is retarded until the occurrence of favorable conditions. However, if early germination occurs, then natural selection would eliminate the unfavorable genotypes in the early stages of their life (Donohue et al. 2005). In the present study, towards the end of the experiment, the seeds displayed faster germination than those that germinated at the beginning of the experiment. This rapid germination coincides with the lack of rainfall that would cause decreased survival of the seedlings of the four species of Asteraceae. It is important to conduct long-term evaluations of the mean germination time on stored seeds since it is known that retarded germination in Arabidopsis thaliana (L.) Heynh. increases its fitness (Donohoue et al. 2005).
Seed longevity and seed viability. It is estimated that seed longevity of the four species of Asteraceae exceeded one year (19 months), considering the age of the seeds from the time of their collection. According to Thompson's classification (Thompson et al. 1997), the studied species could therefore present a short-term persistent soil seed bank (ranging from 1 to 5 years). Although the viability and germination of the seeds decreased with increased longevity, the fact that they can potentially form a short-term seed bank makes development of an ex-situ seed conservation protocol feasible. Within a general pattern of decrease in germination, it is observed a lack of germination uniformity during the period of 375-465 days, when there were alternations between higher and lower germination. This germination asynchrony contributes to the maintenance of populations in seasonal environments (Bhatt et al. 2019). In this study, germination asynchrony was observed in winter months when rain is scarce.
At the other hand, maintenance of viable seeds was similar among the study species. However, it is important to highlight that both A. dentata and S. origanoides were the species with the highest germination percentages, although these values did not exceed 30 %, which is low compared to the average maximum germination percentage (64.4 %) of 40 species of Asteraceae of arid and semiarid environments (Valencia-Díaz & Montaña 2003). For other species of Stevia and Aldama, the maximum germination percentages are 71 and 97 %, respectively (Bertolosi et al. 2015, Uçar et al. 2016). It is possible that the germination percentage observed in this study could increase in substrates that provide higher water availability to the seeds, so that there is a better imbibition to start and complete their germination. The large differences between the germination and viability percentages indicate that germination could be increased: this difference was 30.54 % for A. dentata, 26.21 % for S. origanoides, 27.77 % for V. virgata and 32.5 % for R. barba-johannis. On the other hand, according to Fenner & Thompson (2005), dormancy is the mechanism used by seeds to persist for longer than one year in seed banks. In this case and considering the difference between seed viability and seed germination, the seeds of the studied Asteraceae could present dormancy that allows them to spread the occurrence of their germination over time.
Although the germination percentages of the species were not similar, the behavior in terms of viability, longevity and germination rate did follow a similar pattern. It is important to study the seed biology of useful weed species since this can facilitate the establishment of effective germplasm conservation and/or weed management programs. According to the information generated here, it is suggested to investigate lower seed storage temperatures that may prolong their longevity and avoid loss of viability.











 nueva página del texto (beta)
nueva página del texto (beta)



