Introduction
Listeria monocytogenes is the pathogen responsible for the listeriosis disease that affects human, and it considered a public health problem due to the severity of the diseases and the high mortality rate (20-30 %) that generates in the vulnerable population (Zunabovic et al., 2011). Meningitis, septicemia and abortion are invasive clinical forms of listeriosis, and gastroenteritis is a noninvasive form, both types differ in terms of symptomatology, population, infectious dose and incubation time (Posfay-Barbe & Wald, 2009; Camejo et al., 2011). The main route of infection of the pathogen is by consumption of dairy products with or without pasteurization, sausages, and contaminated vegetables, which leads to listeriosis outbreaks (CDC, 2017; Raheem, 2016).
Given the clinical and epidemiological importance of L. monocytogenes, several methods have been implemented for its detection and typing with the intention of accurately defining the strains involved in outbreaks (CDC, 2017), and to identify contamination sources in the food chain. The Center for Disease Control and Prevention (CDC) of the United States of America, based on the detection and typing of L. monocytogenes, makes it possible to report annually cases of listeriosis (≈1500) associated with food consumption and mortality rate of 17 % (Scallan et al., 2011). In general, developing countries do not consider mandatory report of the listeriosis, a fact that probably affects the low incidence of the disease (Tod & Notermans, 2011).
Several studies refer the presence of L. monocytogenes in foods, and point out the risk they represent in listeriosis foodborne (Adzitey & Huda, 2010). The survival, transmission and pathogenesis of L. monocytogenes is conditioned by the adaption ability of the bacteria (Zunabovic et al., 2011; Roberts & Wiedmann, 2003), the food composition (Midelet-Bourdin et al., 2006), and the diversity among the serotypes of the species (Jaradat & Bhunia, 2003). It is assumed that the 13 serotypes of L. monocytogenes are potentially pathogenic because they possess a chromosomal locus of virulence (prfA, plcA, plcB, hlyA, mpl, acta) called pathogenicity island-1, and invasion genes (Internalinas) required to establish the infective cycle based on adherence, invasion, survival and multiplication within phagocytic and non-phagocytic host cells (Camejo et al., 2011; Vázquez-Boland et al., 2001). However, serotypes 1/2a, 1/2b, and 4b have been identified in most cases of listeriosis (Orsi et al., 2011), so it is important to evaluate whether the strains of food origin independently of the serotype they have certain virulence properties.
Although, in Mexico the presence of L. monocytogenes has been reported in various foods (Castañeda-Ruelas et al., 2013; Silva et al., 2007; Vázquez-Salinas et al., 2001; Saltijeral et al., 1999), there are few clinical cases described, probably due to the fact that listeriosis is not a notifiable disease (Castañeda-Ruelas et al., 2014). In 2016, the General Directorate of Epidemiology of Mexico reported 4,476,041 cases of intestinal infections, 25,896 cases of bacterial food poisoning and 628 cases of meningitis, but the etiology of both clinical cases were not defined (DGE, 2018). However, between 1967-2006, some listeriosis cases were reported, which despite the treatment with antibiotics had a mortality rate of 50 % (Espinoza-Gómez et al., 2006; Castrejon-Alba et al., 1997; Kraus et al., 1994), in none of them was the search for the source and strain of L. monocytogenes responsible for the infection.
A possible explanation of why in Mexico the relationship between listeriosis and its causal agent is not known, possibly due to failures to establish the clinical diagnosis and therefore the etiological diagnosis. On the other hand, although it has worked on the presence of L. monocytogenes in different foods, the information on the isolated strains has been limited to their biochemical identification, so it is not known if they present characteristics that allow establishing the infective cycle in the host. The purpose of the present study was to analyze some virulence properties of strains of L. monocytogenes isolated from food and clinical cases, to define the importance of food in the transmission of virulent strains to humans.
Materials and Methods
Strains
Thirty strains of L. monocytogenes, including 7 strains of clinical cases of listeriosis and 23 strains isolated from food (Table I), previously typed by serotype and pulsed-field gel electrophoresis [PFGE] (Castañeda-Ruelas et al., 2013) were evaluated. Virulence assays included control strains of L. monocytogenes ATCC 7644, and E. coli pathotypes (EAEC-49766, EPEC-2348/69 and EAEC-O42). A bacterial suspension in sterile phosphate buffer (PBS) of 1.0 (≈3x108 cfu/mL) was standardized on the Macfarland scale.
Table 1 Description of strains of L. monocytogenes isolated from food and clinical cases in Mexico.
| Genotype | Serotype | Source | Site isolation | Total strains (n=40) |
|---|---|---|---|---|
| A | 4 b | Raw chicken | Sinaloa | 1 |
| A | N.D. | Clinical | Mexico | 1 |
| C | 1/2 c | Raw meat | Sinaloa | 2 |
| D | 1/2 a | Frankfurters | Sinaloa | 7 |
| F | 4 b | Raw chicken | Sinaloa | 1 |
| G | 4 b | Raw chicken | Sinaloa | 1 |
| H | 4 b | Raw chicken | Sinaloa | 2 |
| I | 4 b | Raw chicken | Sinaloa | 4 |
| J | 1/2 b | Raw meat | Sinaloa | 1 |
| K | 1/2 b | Raw meat | Sinaloa | 1 |
| L | 1/2 b | Raw meat | Sinaloa | 1 |
| M | 1/2 b | Raw chicken | Sinaloa | 1 |
| N | 3 b | Raw meat | Sinaloa | 1 |
| X1 | N.D. | Clinical | Mexico | 1 |
| X3 | N.D. | Clinical | Mexico | 1 |
| X4 | N.D. | Clinical | Mexico | 2 |
| X5 | N.D. | Clinical | Mexico | 1 |
aThe genotype was determined by the pulse field gel electrophoresis method.
Cell culture
The cell lines used were CaCo-2 (human colorectal adenocarcinoma cells), HT-29 (human colon adenocarcinoma cells), and HEp-2 (human laryngeal carcinoma cells). CaCo-2 and HT-29 cells were cultured as monolayers in 75 cm2 flasks with modified Dulbecco Eagle medium (DMEM) supplemented with 10 % (v/v) fetal bovine serum, 1 % non-essential amino acids (v/v) and 1 % (v/v) antibiotic solution (100 μg/mL penicillin and 100 μg/mL streptomycin). HEp-2 cells were cultured in minimal essential medium (MEM) supplemented with 10 % (v/v) fetal bovine serum, 2 mM L-glutamine, and 1 % (v/v) antibiotics. For the adhesion and invasion assays, the cells were cultured in DMEM and MEM without antibiotics for 24 h. The cells were kept at 37 °C under 5 % CO2.
Adhesion assay
The adhesion profile was determined using the HEp-2, HT-29 and Caco-2 cell lines, as described by Cravioto et al. (1991). In cell culture plates with glass lentils, cell monolayers with 95 % confluence (105 cells/well) were cultured and inoculated with strains of L. monocytogenes in multiplicity of infection (MOI) ratio of 10:1. The inoculated plates were incubated at 37 °C for 3 h. The lentils with the infected monolayers were washed with PBS, fixed with methanol, and stained with Giemsa stain. The lentils were examined under the 100x lens with immersion oil in a light microscope. To consider positive the adhesion test, ≥10 bacteria should be counted in the cells contours or surface of the 25 % of the cell monolayers, and the negative control should not show bacteria adhered to the cells. To classify the adherence pattern, control strains EPEC-2348/69, EAEC-49766 and EAEC-O42 were used, which present the localized, aggregative and diffuse pattern, respectively.
Invasion assay
The invasion index was analyzed by the invasion test based on gentamicin in HEp-2 and HT-29 cells as described by Jaradad & Bhunia (2003), with minor modifications. Only those cell lines were selected to evaluate the invasion index of the L. monocytogenes strains because it was not considered necessary to use two cell lines of intestinal origin. Briefly, cell monolayer cultures were grown to a confluence of 95 % (105 cells/well), and inoculated with a MOI of 10:1 with the bacterial strains. The inoculated monolayers were incubated at 37 °C for 90 min; after this time, they were washed with PBS, and were further incubated 90 min at 37° C in DMEM medium supplemented with gentamicin (100 μg/mL) to eliminate the extracellular bacteria. Subsequently, the monolayers were washed with PBS, and the cells were lysed. The quantification of the inoculum and intracellular cells of L. monocytogenes was performed in serial dilutions on Oxford agar, and the value was expressed in Logufc/mL. The invasion index was calculated by dividing the concentration of internalized bacteria (with gentamicin) by the concentration of the inoculum. The strain of L. monocytogenes ATCC 4976 was included as a positive control.
Statistical analysis
The adhesion and invasion tests were performed twice in duplicate for each evaluated strain. For the invasion test, a two-way ANOVA analysis (strains of L. monocytogenes and cell type) was performed completely randomly with the Tukey and Dunnet test. Additionally, the Kruskal-Wallis test was performed to determine the association of the invasion index with the categorical variables such origin, serotype and PFGE. A value p≤0.05 was considered statistically significant (Minitab17).
Results and Discussion
The 30 strains of L. monocytogenes analyzed adhered to HT-29, HEp-2 and Caco-2 cells with a diffuse and/or aggregative adherence pattern (Figure 1), suggesting the ability to colonize and initiate their cycle of invasion in the host. A differential adherence pattern was not observed according to the PFGE profile, origin (clinical and alimentary) and/or serotype among the strains. However, the strains showed different levels of adhesion, damage or destruction of the cell monolayer, and the formation of cellcell extensions (Figure 1).
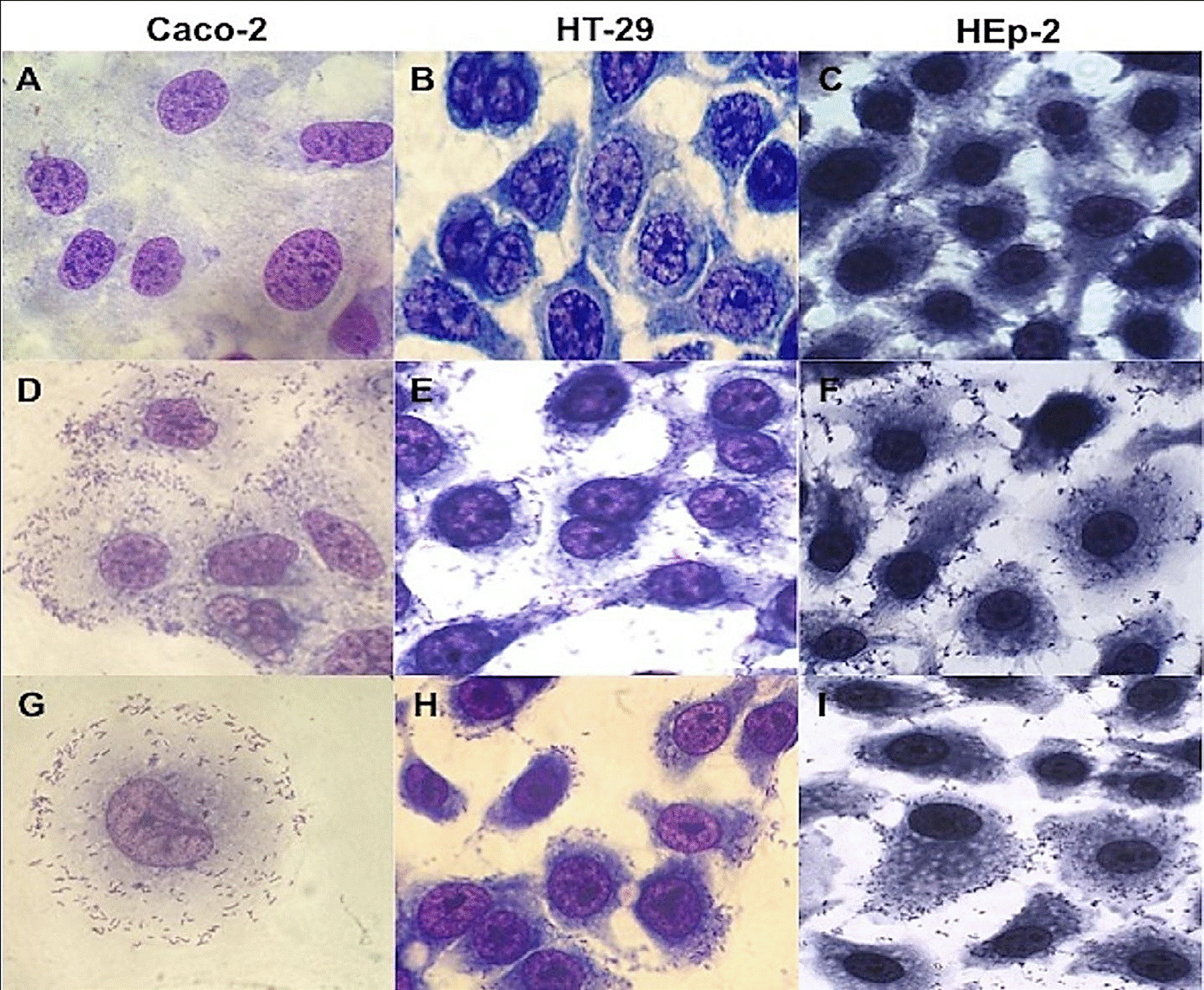
Figure 1 Micrograph of diffuse adherence pattern of L. monocytogenes strains in Hep-2, HT-29 and Caco-2 cells. (A) Caco-2 cell lines control, (B) HT-29 cell lines control, (C) HEp-2 cell lines control, (D) serotype 4b in Caco-2 cells, (E) serotype 1/2c in HT-29 cells, (F) serotype 1/2c in HEp-2 cells, (G) clinical strain in Caco-2 cells, (H), serotype 3b in HT-29 cells, (I) serotype 1/2a in HEp-2 cells. Magnification 100x.
The capacity of L. monocytogenes to invade the HT-29 and HEp-2 cells varied from 0.422-0.865 and from 0.3070.858, respectively, observing statistical differences between strains (p=0.000), cell lines (p=0.001), serotype (p=0.018) and electrophoretic profile (p=0.001), but not with respect to the origin (p=0.658). This indicated that the invasion rate of strains of L. monocytogenes is observed as a non-stable property. The overall invasion profile expressed by the strains of food origin (n=23) was determined with respect to the control strain of L. monocytogenes ATCC 4976 previously reported with pathogenic potential (Jaradat & Bhunia, 2003); the majority (87 %) of the strains corresponds to an invasion index similar to the control strain and the strains of clinical origin. However, a strain corresponding to serotype 1/2a (strain 10) presented an invasion phenotype superior to the strain of L. monocytogenes ATCC 4976. Meanwhile, isolated from serotypes 4b (strain 116) and 1/2c (strain 101) showed a lower phenotype (Figure 2).
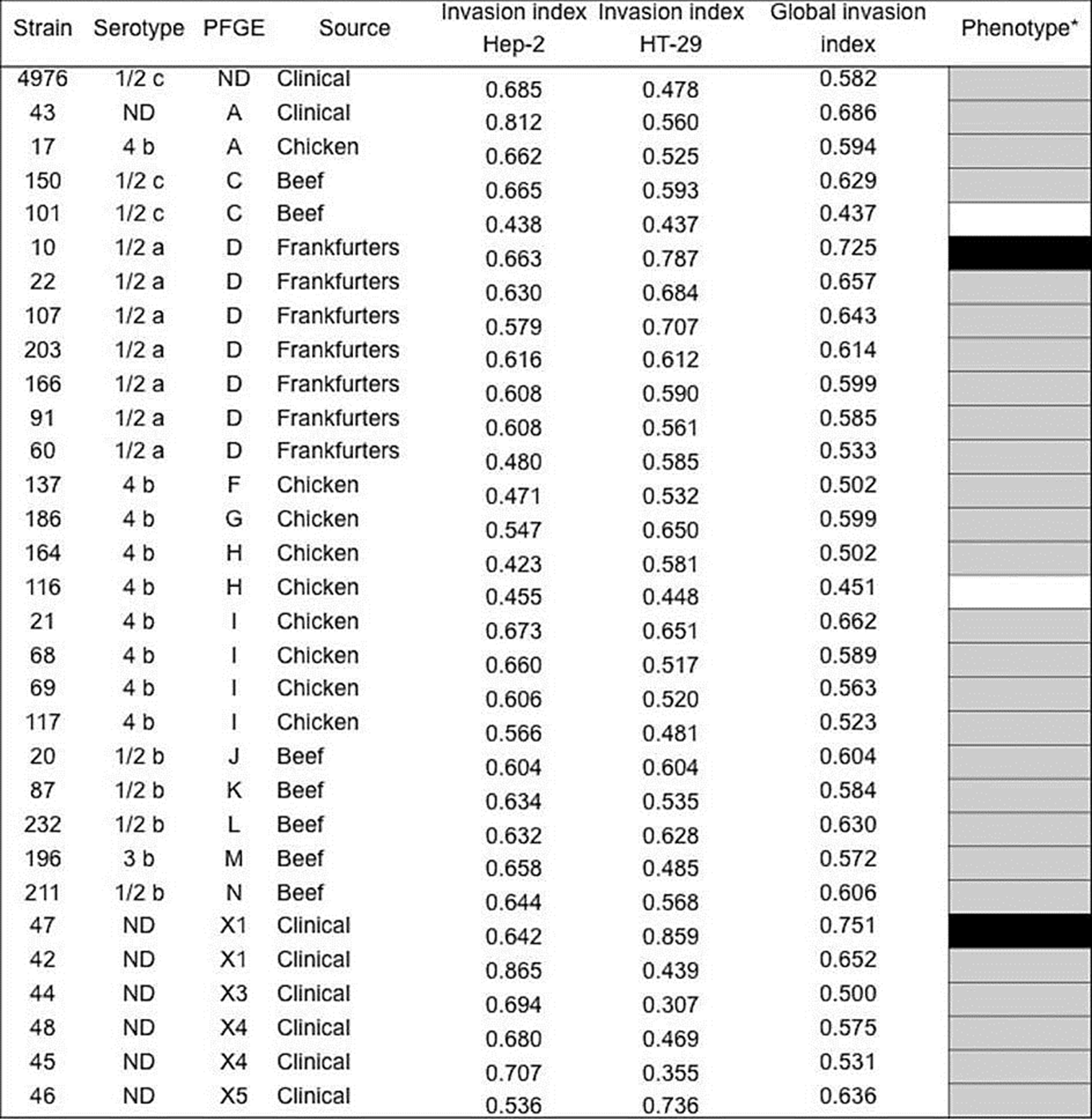
Figure 2 Invasion phenotypes of Listeria monocytogenes strains isolated from clinical cases and food in Mexico. aGlobal invasion phenotype was determined recpect to control strain (L. monocytogenes ATCC4976); higher (black), similar (gray), and low (white) phenotype. The mean of the invasion phenotypes between the strains are statistically different in the HEP-2 and HT-29 cells (p<0.05). N.D: Not determined.
The comparative study of the adhesion and invasion phenotype of L. monocytogenes strains isolated from food and clinical cases, is the first description in this respect that is made in Mexico. In the literature, it is reported that most cases of listeriosis are linked to serotypes 1/2a, 1/2b and 4b, and their transmission is through food (Orsi et al., 2011). Our study shows that most of the strains of food origin belong to these serotypes with potential pathogenic capacity in vitro in cell lines.
To initiate the event of invasion of host cells and ensure the progression of the intracellular cycle of L. monocytogenes, adherence is a primordial process (Camejo et al., 2011; Vázquez-Boland et al., 2001). Some studies have quantified the adhesion capacity of L. monocytogenes, finding differences in the degree of expression according to the serotype (Jaradat & Bhunia, 2003). However, information on the description of the topology of the host cell infected with L. monocytogenes is scarce. In this study it was identified that the strains of L. monocytogenes present a diffuse adherence pattern similar to that described in E. coli (Nataro & Kaper, 1998), which showed no relation with the serotype and origin of the bacteria or with the cell epithelial assays.
In the model of polarized epithelial cells it has been observed that the invasion of the pathogen is carried out by a basolateral adhesion, which is the result of the interaction between the bacterial surface proteins (Internalinas) and the E-cadherin protein that surrounds the junctions of the host cell (Pentecost et al., 2006). In this sense, the diffuse phenotype of the evaluated strains is related to this description, and to the influence of the bacteria on the type of host cell infected when showing cell damage and cell-cell extensions. L. monocytogenes can cause cellular damage due to the invasion effect or potential toxins production, both pathogenic properties characteristic of the bacteria (Camejo et al., 2011; Pentecost et al., 2006; Vázquez-Boland et al., 2001). Scalestky et al. (2002) described the presence of different adhesion phenotypes and their relationship with the clinical manifestations induced by E. coli, which is a highly diversified microorganism, a fact that contrasts with that observed with L. monocytogenes that does not present differential adhesion phenotypes.
Previously in Mexico, Vázquez-Salinas et al. (2001) identified isolated strains of food with serotypes 4 and 1/2, which in a murine model were identified as nonvirulent and virulent bacteria, respectively. In contrast, in the present study when evaluating the invasion degree of the food origin strains, it was observed differences between strains/serotypes/PFGE analysis, which allows to conclude that the virulence of L. monocytogenes is an individual property that varies between the different strains. In this sense, some studies have described the non-relationship of the invasion degree with the expression of adhesion or hemolysis of L. monocytogenes strains and/or serotypes (Jaradat & Buhnia, 2003). Rychli et al. (2014) point out that genomes of different strains of L. monocytogenes maintain a similar group of genes of functional virulence (pathogenicity-1 island) allowing the infective cycle, but, distinctive virulence genes of each strain reflect the virulence magnitude. Additionally, greater affinity of L. monocytogenes was observed by HT-29 cells, this could be attributed to the intestinal origin of the cells and the ability of the pathogen to cause gastrointestinal diseases (Posfay-Barbe & Wald, 2009).
According to other studies, our results confirmed that L. monocytogenes independent of its origin are pathogens with different virulence, and that this may be conditioned by the serotype or its genetic profile (Indrawattana et al., 2011; Larsen et al., 2002). Information in this regard indicates that some strains of L. monocytogenes isolated from food may have a limited human-pathogen potential (Roberts et al., 2005), and that in certain cases it depends on the matrix and storage temperature of the food (Midelet-Bourdin et al., 2006), or it is related to additional molecular factors that contribute to the pathogenic cycle (Roberts & Wiedmann, 2003). Although, with some differences due to the invasive capacity observed in the food source and clinical strains, the participation of food as a source of transmission of potentially pathogenic L. monocytogenes clones responsible for Listeriosis in Mexico may be proposed.
Conclusions
In Mexico, L. monocytogenes is not a pathogen of mandatory diagnosis in suspected clinical cases. However, there are reports of sporadic cases and studies that support the prevalence of the pathogen in food. Our results showed that most serotypes of L. monocytogenes isolated from food have the ability to adhere to and invade host cells similar to the behavior of clinical strains, and that food represents a major route of transmission. Given this, there is a need to implement measures to carry out stricter control of food, through the intentional search for L. monocytogenes as a foodborne pathogen in Mexico. On the other hand, it is important to ask the doctors that in the clinical cases of abortions or neurological problems in which the diagnosis is not established, it is convenient to consider the possible participation of the bacteria. The molecular studies of the bacteria are shown as a promising possibility, to develop systems for rapid identification and to learn more about the genomics of the microorganism.











 texto en
texto en 


