Introduction
Given that deforestation, habitat modification, and degree of human contact with wild animals are increasing exponentially in most parts of the world (Chapman et al. 2006), several studies have explored how these factors may impact on parasite infection risk in non-human primates (Clarke et al. 2002; Kowalewski and Gillespie 2009; Cristóbal-Azkarate et al. 2010). However, the studies focusing on the relationship between parasite prevalence and richness with life history variables such as individual age and sex in wild primates are scarce (MacIntosh et al. 2010; Friant et al. 2016).
The age of an individual has been reported to differentially affect rates of parasitic transmission in several vertebrate taxa including fish, birds, bats, rodents, and non-human and human primates (Krasnov et al. 2006; Plowright et al. 2008; MacIntosh et al. 2010). In the case of wild primates, studies have shown that when age increases, parasitism could also increase if, for example, larger-bodied individuals occupy more space, require more resources and have contact with contaminated foods and substrates disproportionately (Hudson and Dobson 1997). In fact, studies of non-human primates found that adults had higher helminth parasite infection rates than juveniles (e. g., Cebus capucinus, Parr et al. 2013; Alouatta pigra, Eckert et al. 2006; Mandrillus sphinx, Setchell et al. 2007). On the other hand, lack of acquired immunity in younger individuals may increase risk of parasitism in juveniles (Hudson and Dobson 1997), given that younger individuals require constant exposure to pathogens to stimulate their immune system to develop antibodies to limit subsequent pathogenic infections during adulthood (Lloyd 1995). For example, a study in Mexico reported that juveniles Alouatta palliata showed a 1.6-fold higher helminth and protozoan parasite prevalence than adults (Stoner and González Di Pierro 2006). However, other studies reported no differences between helminth and protozoa parasite infection and age classes (e. g., A. palliata, Maldonado-López et al. 2014; A. pigra, Trejo-Macías and Estrada 2012; Colobus vellerosus, Teichroeb et al. 2009).
Regarding sex, parasitism tends to be more common in males than in females across vertebrate taxa, including humans (Klein 2004; Habig and Archie 2015). Males generally invest most of their effort into attaining and maintaining high rank and central positions in non-human primate species (Zuk and Stoehr 2002). In this regard testosterone facilitate the achievement of a high rank but there are a number of costs imposed by elevated levels of this hormone, such as immunosuppressive effects, increasing the risk of acquiring parasitic infections (tradeoffs hypothesis; Muehlenbein and Bribiescas 2005): This idea was tested in a study on adult male chimpanzees (Pan troglodytes) at Ngogo, Uganda, where high ranking males had higher testosterone levels and an increased intestinal helminth burden but not protozoan, when compared to lower male ranking animals (Muehlenbein and Watts 2010). In addition, studies in non-human primates, determined that immunosuppressive effects of stress hormones also could increase susceptibility in either dominant or subordinate individuals depending on species-typical dynamics and hierarchical stability (stress-response hypothesis; Cavigelli and Caruso 2015; Sapolsky 2005). Nevertheless, in Japanese macaques, Macaca fuscata, for example, socially mediated exposure seems to be more important than the immunosuppressive effects of stress in explaining why dominant females have more infections from directly transmitted parasites (MacIntosh et al. 2012). These studies, therefore, show that the relationship between infection patterns and intrinsic factors of the host need further research (Nunn and Altizer 2006) and consider that infection patterns largely depend on the level of exposure of the host to the infectious stages of the parasites, to the physiological factor, and the social dynamics of the group studied. For the first time, we investigated the relationship between Giardia spp. and Blastocystis spp. prevalence and age and sex categories in groups of black and gold howler monkeys (Alouatta caraya) that inhabit fragmented forests in Northern Argentina. Field studies conducted on wild populations of A. caraya in Argentina have shown that zoonotic protozoa as Giardia spp. and Blastocystis spp. are present and prevalent in wild black and gold howlers, therefore, these protozoa are a natural component of the howler parasite communities (Venturini et al. 2003; Kowalewski et al. 2011; Milozzi et al. 2012). These protozoa have a direct life cycle and are also the most commonly reported parasite in humans and both wild and domestic animals (dogs, cats, sheep, goats, cows, pigs, horses, among others), in both cases, transmission can occur through ingestion of infective stages (cysts), and human infection is associated with poor sanitary conditions, contact with animals and consumption of contaminated food or water (Godoy et al. 2004).
Materials and Methods
Study site and studied groups. The A. caraya groups studied inhabit extensions of semideciduous gallery forests around the Estación Biológica Corrientes and San Cayetano Provincial Park (of 78 ha; -27° 33’ 09.5” S, -58° 40’ 48.2” W) in the northwest of Corrientes province in Argentina (Figure 1a). These forests have been strongly modified by logging, burning and the presence of livestock, and households are distributed throughout this rural site (Kowalewski et al. 2011). The climate is subtropical, with an average annual temperature of 21 °C and an average annual precipitation of 1,200 mm (Rumiz et al. 1986). Rains increase slightly towards the spring and summer seasons (September to December).
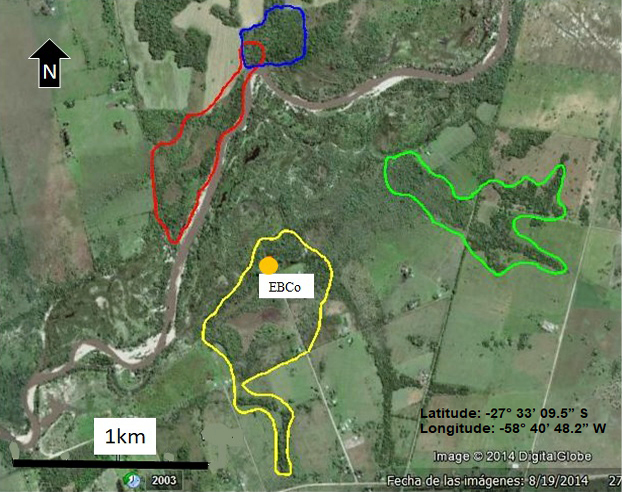

Figure 1 a) Location of area of study in Corrientes, Argentina and b) Area of action of the four studied groups. Blue (group 1), red (group 2), yellow (group 3), green (group 4).
Fecal samples were collected from 27 individuals (13 juveniles and 14 adults) of both sexes (13 males and 14 females) belonging to four groups of howler monkeys. A subset of adult individuals was sampled, and all juvenile individuals. Figure 1b depicts the home range of the four study groups.
We categorized juveniles in two age categories: category 1 (from 1 to 2.5 years of age) and category 2 (more than 2.5 to 4 years of age). The age-sex category composition of each of the study groups is in Table 1.
Table 1 Distribution of the number of individuals according to sex and age category in each studied group in Corrientes, Argentina.
| Groups | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Total (Individuals) | 6 | 6 | 8 | 7 | |
| Adults | Males | 1 | 2 | 2 | 1 |
| Females | 2 | 2 | 2 | 2 | |
| Juveniles | Males category 1 | - | 1 | 2 | 1 |
| Males category 2 | 1 | 1 | - | 1 | |
| Females category 1 | 1 | - | 1 | 1 | |
| Females category 2 | 1 | - | 1 | 1 |
Sample collection and examination. Fecal samples were monthly collected during the morning between August 2014 and September 2015, immediately after defecation to minimize the risk of contamination. Only the central portion of the fecal sample was taken using disposable wooden spatulas. Samples were stored individually in 20 ml flasks with 10 % formalin and each flask was shaken to homogenize the sample with formalin (Gillespie 2006; Gillespie et al. 2008) and then labeled and stored with the date of collection, observer, location and identification of individuals.
Samples were examined in the Laboratorio de Biología de los Parásitos of the Facultad de Ciencias Exactas y Naturales y Agrimensura, during March-December 2017, using microscopy and techniques of flotation (Sheather’s solution, D = 1.27; Milozzi et al. 2012) and sedimentation (1g of feces), as per Gillespie (2006) to ensure the collection of cysts of both protozoa (Figure 2a, 2b). In each technique, slides (18 mm x 18 mm) were examined under a stereoscopic magnifying glass (Olympus CH30; x400 magnification), previously colored with a drop of Lugol’s solution; all samples were examined in duplicate.
Data analysis. We described parasite infections in terms of prevalence of infection. Prevalence is the proportion of individuals hosts sampled infected with a particular parasite species (Stuart and Strier 1995; Bush et al. 1997; Gillespie 2006).
To analyze the relationship between age, sex and infection prevalence, a Generalized Linear Mixed Model (GLMM) was used with Gaussian family and link function ‘’Identity’’, which considered prevalence (0.5 ± 0.06) as a variable response and age (with three levels: juvenile 1, juvenile 2, adult) and sex (with two levels: male, female) as fixed effects. Additionally, the individual nested in group was considered as a random effect. The adjustment of the model was evaluated using a maximum likelihood ratio test (LRT) where we compared models with variations in a fixed effect to take into account all the comparisons the random effects are the same (individual nested in group; Bolker et al. 2009). All statistical analyses were performed in R through the R-studio platform, version 3.2.1 (R Core Team 2016). Statistical significance was set at 0.05 for all interpretations.
Results
A total of 375 samples were collected, with at least three samples (of different days) per individual per month from 27 individuals (13 juveniles and 14 adults), of both sexes (13 males and 14 females) belonging to four groups of howler monkeys.
Parasite prevalence for both protozoan taxa was 88.9 % (24/27). Of the 27 individuals analyzed, we found Giardia spp. infection prevalence to be 81.4 % (22/27) and Blastocystis spp. infection prevalence to be 77.7% (21/27).
In adults, infection prevalence was 78.5 % (11/14) while in juveniles, all individuals (juveniles 1 and juveniles 2; 100%) seemed to be infected with both protozoa. Males had a prevalence of general infection 84.6 % (11/13) and females had a 92.8 % (13/14; Table 2). No significant effects of age or sex on prevalence was found as the model selected by LRT was null (P > 0.05 for sex and age; Figure 3a, 3b).
Tabla 2 Prevalence of infection according to sex/age categories in four groups of A. caraya in Corrientes, Argentina (n = number of Individuals analyzed).
| Sex/age categories | n | Prevalence of Infection (%) | |||
|---|---|---|---|---|---|
| General | Giardia spp. | Blastocystis spp. | |||
| Total | 27 | 88.9 | 81.4 | 77.7 | |
| Adults | Males | 13 | 84.6 | 84.6 | 76.9 |
| Females | 14 | 92.8 | 78.5 | 78.5 | |
| Total | 14 | 78.5 | 64.2 | 71.4 | |
| Juveniles | Males | 6 | 66.6 | 66.6 | 66.6 |
| Females | 8 | 87.7 | 62.5 | 75.0 | |
| Total | 13 | 100 | 100 | 84.6 | |
| Males category 1 | 4 | 100 | 100 | 75.0 | |
| Males category 2 | 3 | 100 | 100 | 66.6 | |
| Females category 1 | 3 | 100 | 100 | 100 | |
| Females category 2 | 3 | 100 | 100 | 100 |
Discussion
The goal of this research was to explore if Giardia spp. and Blastocystis spp. protozoa parasite infection rates (estimated through infection prevalence) were affected by sex-age in four groups of black and gold howler monkeys (A. caraya) living in forest fragments. Our results suggest that infection prevalence of these protozoans is not affected by these biological factors. These findings are consistent with other studies that also have examined whether host intrinsic traits (age-sex) affect infection gastrointestinal parasites in primates. For example, a study based on 982 stool specimens collected from adult and juvenile individuals from a multimale-multifemale social group of red-capped mangabeys (Cercocebus torquatus) in Nigeria, shows that the acquisition of protozoan infections did not vary according to host traits (Friant et al. 2016). Other studies, conducted over 6 to 7 months by Vitazkova and Wade (2007) and Trejo-Macías and Estrada (2012), based on < 200 fecal samples, did not find significant differences in helminth and protozoan parasite prevalence among adults, juveniles, and infants. On the other hand, Stoner and González Di Pierro (2006) reported that juveniles from three groups showed a 1.6-fold higher helminth and protozoan parasite prevalence than adult howler monkeys (A. pigra) in Montes Azules, México. In short, these set of studies indicate, that there is a general tendency for protozoa to be acquired in a uniform way in primate groups.
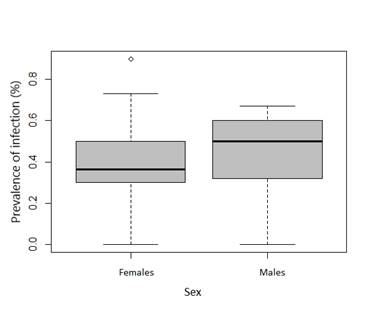

Figure 3 Box plots showing infection prevalence according to age (a) and sex (b) in Alouatta caraya. Median (black line), interquartile ranges (rectangle) and minimum and maximum values (whiskers).
Recent research suggests that parasite infection rates may be influenced by specific form of transmission of different parasites (Día 2001; Nunn and Altizer 2006). Then, transmission of these protozoa may occur through ingestion of infective stages (cysts) that do not require develop in the external environment for days to months before they become infective like helminths (Freeland 1980), thus, they are immediately infective once defecation has occurred and can survive in the environment for weeks or months (Godoy et al. 2004). Moreover, all members in social group tend to defecate simultaneously in their trees after periods of resting (Gilbert 1997; Kowalewski and Zunino 2005), this defecation pattern contributes to the presence of areas of vegetation (latrines) contaminated with clumped feces (potential sources of infection) within the home range of howler groups (Van Belle and Estrada 2006) leaving all members of the group exposed to infection. Therefore, we suggest that physiological or behavioral factors related to the risk of parasitic infection (i. e., that tend to vary according to age and sex in primates) do not appear to be important in the risk of protozoan infection.
It is noteworthy that in our study a high general prevalence of infection was found (88.9 %), that is, 24 out of 27 individuals presented at least one or both protozoa. Our study area is under continued deforestation due to selective logging and cattle ranching, such levels of deforestation therefore, decrease habitat size, forcing all howler monkeys to descend to the ground and cross from fragment to fragment looking for supplemental food resources (Zunino et al. 2007) potentially increasing contact with parasites on the ground and in small water bodies (Kowalewski and Gillespie 2009). Additionally, cattle enter into the forest fragments opening trails and defecating along them, also, drink and defecate in streams where as well black and gold howlers drink water, thus increasing the chances of infection in the entire population (Kowalewski et al. 2011). These forest systems may explain our high infection rates for the study protozoans (Bublitz et al. 2015).
Although hypotheses are established that predict possible biases in wild primate populations in relation to intrinsic variables of the host such as sex and age, it is important to consider that host-parasite relationships are highly specific and vary among populations (Hudson and Dobson 1997). Therefore, we consider that studies designed to examine age-sex effects need to consider other potential infection risk factors, such as habitat disturbance (e. g., logging rates, Gillespie and Chapman 2008), parasite life history and transmission, especially in non-human primate populations that are constantly subjected to the reduction of their habitats (Chapman et al. 2006).











 nueva página del texto (beta)
nueva página del texto (beta)



