Introduction
Global warming has been considered one of the major contemporary threats to biodiversity and survival of terrestrial ectothermic species in the tropics (Huey and Tewksbury, 2009, Williams et al., 2007), where most of these organisms operate at environmental temperatures closer to their thermal tolerance limits for suitable performance of key physiological processes such as feeding, water balance, locomotion and breeding (Andrews, Mathies, & Warner, 2000). Therefore, even a slight temperature increase could lead the local extirpation of populations (Huey and Tewksbury, 2009, Sinervo et al., 2010). Furthermore, some ectothermic species such as lizards exhibit low dispersion capacities to migrate in short term to localities with suitable thermal conditions (Chen, Hill, Ohlemüller, Roy, & Thomas, 2011). In consequence, lizards in tropic latitudes could be more vulnerable to the effects of global warming than species of temperate latitudes (Deutsch et al., 2008, Huey et al., 2009).
However, before considering the inexorable negative effects of rising temperatures expected on the persistence of populations, it is important to regard that some populations might have experienced extreme heat for over several decades during their respective reproductive periods. The fact that such populations remain, shows that lizards are able to avoid deleterious effects of damaging temperatures on developing offspring and themselves, possibly by means of using thermal heterogeneity of microhabitat behaviorally (Huey et al., 2012, Scheffers et al., 2014). Thus, 2 main questions arise: (1) which are the specific behavioral mechanisms that have enabled small Sceloporus lizards to achieve and maintain suitable temperature ranges for their developing offspring and themselves? and (2) can behavioral mechanisms be useful to lizard populations persistence in a global warming context? Therefore, a main goal in the short term should be to describe and understand how species have used the thermal heterogeneity at the microhabitat level and how these have allowed them to deal with historic high macroclimatic thermal regimes. We hypothesize that both viviparous and oviparous sceloporine tropical lizards attain suitable body temperatures for their offspring, modulating specific thermoregulatory mechanism as microhabitat selection and shuttling between microhabitats in the case of viviparous species, and accurate selection of nesting sites of oviparous species. To test the above ideas may allow us to generate inferences on their future persistence and distribution in a global warming context.
Here, we aimed to assess whether 2 lizard species, with different parity mode, have historically been exposed to temperatures close or above their thermal tolerance limits for a successful embryonic development (34 °C; Beuchat, 1986, Mathies and Andrews, 1997), and if so, which behavioral mechanisms have enabled them to face these thermal regimes. For viviparous Sceloporus stejnegeri, we characterized the current operative temperature (Te) available, as well as relevant traits of its thermoregulatory behavior. For oviparous Sceloporus horridus, we characterized the temperature available at potential nesting sites. By using 2 species with different reproductive modes allowed us to understand how these species have avoided overheating and discuss if behavioral abilities such as thermoregulatory traits and nesting site selection will enable them to successfully face heat stress in a future scenario of temperature rise.
Materials and methods
Viviparous lizard Sceloporus stejnegeri (Stejneger's spiny lizard) breeds from fall to winter. Studied population inhabit the tropical dry forest at 350 m in elevation (type locality) at Tierra Colorada, Guerrero (16°27′36″ N, −98°39′ W) (Bell et al., 2003, Wiens and Reeder, 1997). Oviparous S. horridus (Southern rough lizard), breeds from spring to summer. Our study population is located in tropical dry forest at 520 m asl (Sites et al., 1992, Valencia-Limón et al., 2014) at Xalitla, Guerrero (17°59′24″ N, −99°32′24″ W). Both localities are located in the middle section of the Balsas River basin. Embryonic development of both species tolerated a maximum thermal threshold of 34 °C under laboratory treatments (Beuchat, 1986, Mathies and Andrews, 1997) and when this boundary is exceeded, embryos may exhibit physical abnormalities which increase their mortality (Beuchat, 1988, Beuchat and Ellner, 1987).
We obtained and analyzed the trend of historical maximum environmental temperature (Thmax) for both localities during the reproductive season of each studied species: from October to March for S. stejnegeri (Ramírez-Pinilla, Calderón-Espinosa, Flores-Villela, Muñoz-Alonso, & Méndez-de la Cruz, 2009) in Tierra Colorada and from March to September for S. horridus in Xalitla (Valdéz-González & Ramírez-Bautista, 2002). Historical maximum environmental temperature values (Thmax) were obtained from 3 meteorological stations situated near (around 1 km) each locality (Comisión Nacional del Agua [Conagua] http://smn.cna.gob.mx/es/climatologia/informacion-climatologica; temperature records ranging from 1953 to 2000). With these data we are able to sketch the macroclimatic temperature regime experienced by both lizard populations over the past decades in each locality.
Values of maximum environmental temperature (Tfmax) forecasted by using climate model outputs were obtained, from the Centro de Ciencias de la Atmósfera (Universidad Nacional Autónoma de México [UNAM]; http://www.atmosfera.unam.mx). Climate projections were derived for 2030 and 2050 ECHAM5/MPI climate model. Climate anomalies were scaled based on the A2 scenario, which describes a heterogeneous world regionally oriented to the economic development. The per capita economic growth and technological change are slower than in the other scenarios. Global concentrations of CO2 shall increase from 380 ppm in 2000 to 700 ppm in 2080, and temperature will rise by 2.8 °C according to suggested by the Intergovernmental Panel on Climate Change (IPCC, 2013).
Fieldwork in Xalitla in July was carried out, which corresponds to the organogenesis stage development for S. horridus embryos (Valdéz-González & Ramírez-Bautista, 2002). Because it is difficult to locate lizard nests, we characterized soil temperature for several depths of potential nesting. Egg temperature is regulated by the temperature of the surrounding soil therefore, measurement of soil temperature represents a good proxy for the temperature experienced by embryos within the nest (Ackerman & Lott, 2004). Potential nesting places were located under canopy covered areas (i.e. shade conditions) where gravid adult females were observed, and at contiguous open sandy areas (i.e. full sun conditions) potentially used by nesting females (Angilletta, Sears, & Pringle, 2009). Potential nesting places were separated by 5 lineal meters from each other.
We dug 18 nests perpendicularly to ground surface and set Dataloggers (Hobo pendant® UA-002-08; 0–50 °C ± 0.53 °C at 0–50 °C calibration) in each hypothetical nest. Datalogger sensors were placed as follows: 6 inside hypothetic nests at the known nesting depth under shade microhabitats (4.7 cm; Warner & Andrews, 2002), 6 inside hypothetic nests at the known nesting depth under full sun conditions (6.4 cm; Angilletta et al., 2009), and finally 6 inside hypothetical nests built at experimental depths (8.4 cm) under full sun. Further, one Datalogger was used to record current air temperature (Tcenv) at 1 cm above the ground in sun-exposed sites. Temperature was recorded every 30 min from 08:00 to 18:00 h for 6 consecutive days in July. We compared temperatures between nest in shaded and full sun microhabitats and among nests depths via nested Anova test, because both variables are changed at the same time (Logan, 2011). Additionally, we used a linear regression between nests at 6.4 cm in depth and Tcenv to test whether there is a relationship between them, as a way to connect nest temperature data with historical temperature data.
Fieldwork in Tierra Colorada was carried out in November, which corresponds to organogenesis stage development of embryos for S. stejnegeri (Ramírez-Pinilla et al., 2009). To record operative temperature (Te) experienced by adult females, we built hollow copper models (85 mm and 31.8 mm in diameter) that mimic the morphology and absorptivity of S. stejnegeri lizards. Models were calibrated outdoors in the Tierra Colorada locality using restrained live lizards to observe if their Tb and temperature of models varies jointly and attains the same equilibrium temperatures. We measured simultaneously, body temperature (Tb) of 3 restrained live lizards and 3 copper models temperatures attached to a digital thermocouple thermometer. A pair composed of a copper model and a restrained live lizard were placed under solar radiation loads available at each of the microhabitats deep shade (Dsh), filtered sun (FS) and complete sun (CS), we simultaneously measured both temperatures every 60 s for 10 min, during midday when the heat loads tend to reach their maximum intensity in the Tierra Colorada locality.
We validated if model temperatures accurately simulate Tb of lizards by a regression analysis (a significant correlation between Te models and Tb lizard was detected F1, 9 = 177.58; R2 = 0.957; p = <0.001 in Dsh; F1, 9 = 72.90; R2 = 0.901; p = <0.001 in FS and F1, 9 = 112.19; R2 = 0.933; p = <0.001 in CS). This procedure is appropriate for small animals [≤30 g and100 mm of snout-vent length (SVL)] with negligible heat capacity (Bakken, 1992, Bauwens et al., 1996, Díaz, 1997, Dzialowski, 2005, Hertz et al., 1993). After calibration, we plugged an external thermocouple Hobo U23 Pro v2 external temperature® Datalogger (−40 to 70 °C ± 0.2 °C at 0° to 50 °C calibration) to the copper model's central cavity (Dzialowski, 2005, Hertz, 1992, Hertz et al., 1993). In each microhabitat 10 copper models (n = 30) were placed, with the same location and orientation when lizards were observed in the field (see Grant and Dunham, 1988, Hertz, 1992). Then, we recorded every 30 min from 08:00 to 18:00 h for 6 consecutive days (Bakken, 1992). Current air temperature (Tcenv) at 1 cm above the ground was also recorded with one Datalogger in CS microhabitat. Both, female gravid and viviparous pregnant lizards were captured to record their Tb using a Miller and Weber® cloacal rapid reading thermometer Schultheis model (0–50 °C). We compared Te values between microhabitats with a non-parametrical analysis (Kruskal–Wallis test) using localities and microhabitats as factors.
Additionally, when an individual was sighted out of their shelter the following thermoregulatory traits were registered: (1) microhabitat occupied to determine which is the most frequented by lizards, and (2) basking time (BT), in seconds, that lizards spent in a specific microhabitat, which was compared between microhabitats via one-way Anova. Chi square test with Monte Carlo simulations (5,000) and post hoc Marascuilo procedure was used to test significant differences in the frequency in which active lizards were observed on each microhabitat (Agresti, 1990). Statistical analyses were implemented with α of 0.05 in R (R Development Core Team, 2013).
Results
For both study sites, we found that Thmax was higher than 34 °C for most of the months, during the reproductive season for both species, in the last 50 years. For S. stejegeri warmer months were November–March and for S. horridus were March–August (Fig. 1). In Tierra Colorada, Thmax average was 34.4 ± 0.23 °C and did not show statistical differences (F2, 400 = 2.38; p = 0.093) with maximum forecasted environmental temperature (Tfmax) to 2030 (
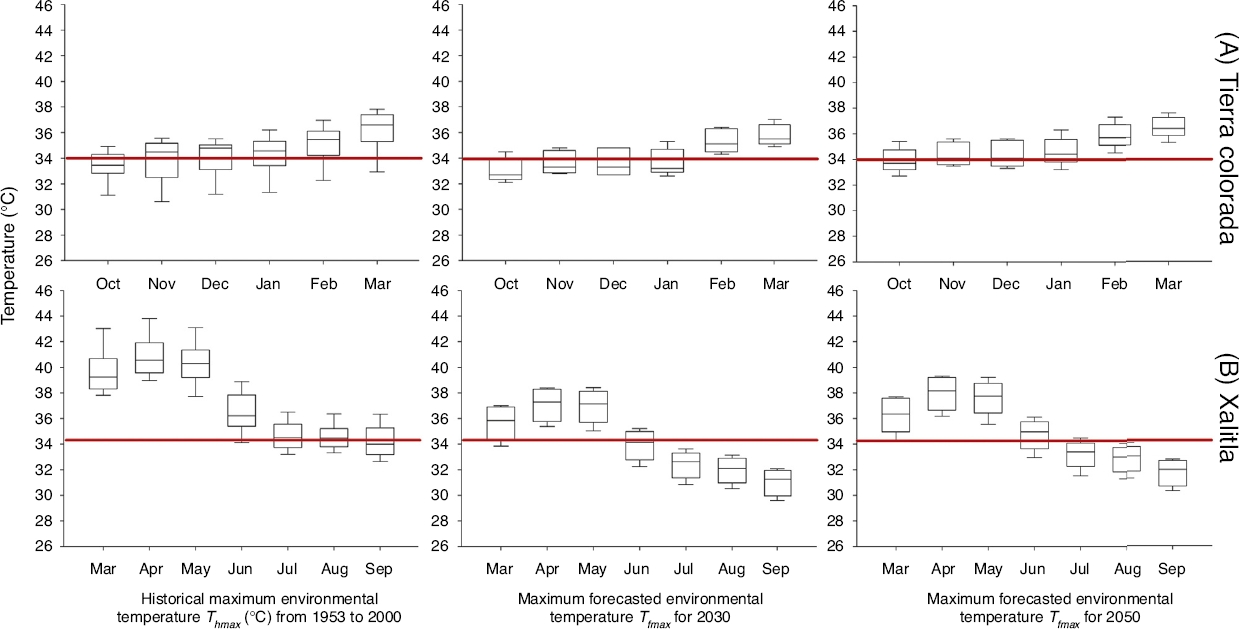
Figure 1 Historic mean monthly maximum environmental temperatures available in the localities inhabit by Sceloporus stejnegeri in Tierra Colorada (A) and S. horridus in Xalitla (B), in their respective reproductive season months from 1953 to 2000 and forecasts for 2030 and 2050. The red line shows the critical thermal maximum for developing embryos.
In Tierra Colorada, we found statistical differences for current operative temperature (Te) at microhabitat level (H2 = 9.961; p < 0.05, n = 33), where lowest values were recorded in Dsh (
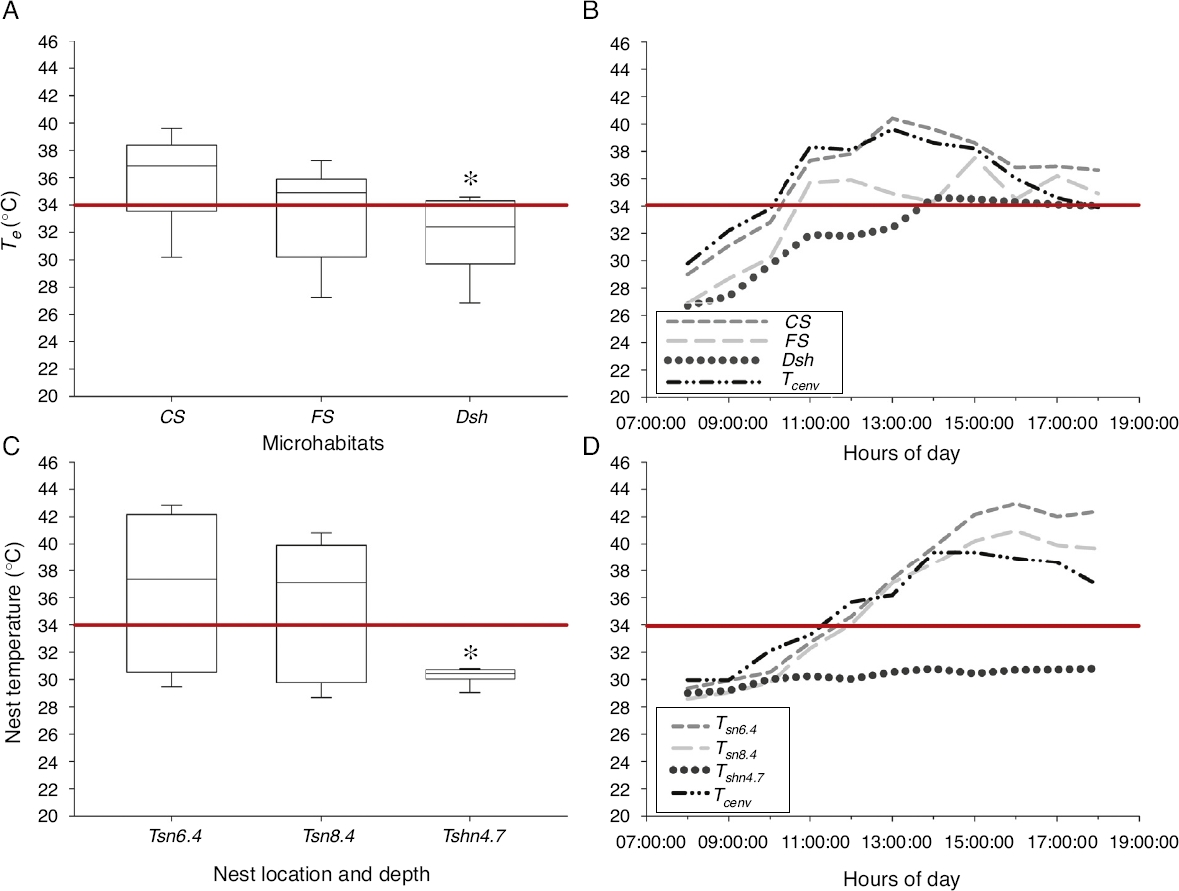
Figure 2 (A) Operative temperatures (Te) measured at complete sun (CS), filtered sun (FS) and deep shade (Dsh) microhabitats; (B) current air (Tcenv) and microhabitats temperature variation through the day at each microhabitat used by pregnant Sceloporus stejnegeri lizards at Tierra Colorada, Guerrero; (C) representative temperatures at different soil depths in full sun and shade conditions, and (D) current air temperature (Tcenv) and nest variation through the day for Sceloporus horridus lizards in Xalitla, Guerrero. The red line is the critical thermal maximum to development embryos of both Sceloporus lizards’ species (34 °C). Asterisk denotes significance at p < 0.05 level.
For basking time of S. stejnegeri we observed active individuals spending significantly more time in Dsh (
Table 1 Absolute differences and critical values that denote statistically significant when absolute difference is greater that the critical range, between pairs of microhabitats where lizard's proportions were evaluated by Marascuilo procedure, implemented for positive Chi-square test.
| Microhabitats contrast | Absolute differences | Critical value | Significant |
|---|---|---|---|
| |p(Dsh) − p(FS)| | 0.5000 | 0.3329 | Yes |
| |p(Dsh) − p(CS)| | 0.6000 | 0.2998 | Yes |
| |p(FS) − p(CS)| | 0.1000 | 0.2737 | No |
At Xalitla, current air temperature (Tcenv) determines heavily the temperature available in artificial nests at 6.4 cm depth, according to linear regression analysis (F1, 131 = 356.76; R2 = 0.733; p < 0.05). Also, the current temperature values in nests built in shade microhabitat at 4.7 cm depth (
Discussion
Ectothermic species can show different biological responses to climate change, from hereditary genetic changes (Bradshaw and Holzapfel, 2006, Logan et al., 2014, Moritz et al., 2012) to phenotypic plasticity (Pigliucci, Murren, & Schlichting, 2006). The evidence in literature suggests that the most straightforward modification under climate change could be behavioral issues. For example, switching time of activity and choosing cool shaded microhabitats as thermal refuges could prevent lizards from being exposed to lethal temperatures (Huey et al., 2003, Kearney, 2013, Kearney et al., 2009, Scheffers et al., 2014, Sunday et al., 2014).
Although adults of some species belonging to the Sceloporus genus might tolerate temperatures up to 40 °C (Angilletta et al., 2013, Clusella-Trullas et al., 2011, Clusella-Trullas and Chown, 2014), here we focused on thermal threshold for a successfully embryonic development (34 °C; Beuchat, 1986, Mathies and Andrews, 1997). Our analyses of historical macroclimatic temperature showed that maximum temperature has frequently exceeded the tolerated threshold of 34 °C for successful embryonic development for both Sceloporus lizard species. This fact suggests that S. horridus and S. stejnegeri have effectively used the thermal heterogeneity available at microhabitat level to avoid lethal overheating. This heterogeneity is provided by vegetation and local orography, which has enabled them to avoid the potential lethal effects of high environmental temperatures via regulating behavioral traits to attain suitable temperature ranges to their developing offspring (Goller et al., 2014, Sears et al., 2011).
We found that pregnant viviparous S. stejnegeri lizards attained a suitable temperature range for themselves and their offspring mainly by choosing microhabitats with cooler conditions under canopy, moreover, rocky substrates with crevices available would be useful for lizards to avoid the exposure to lethal temperatures in the warmest microhabitats for longer times (Scheffers et al., 2014, Sears et al., 2011). On the other hand, oviparous S. horridus might be choosing potential nesting sites with appropriate thermal conditions to lay their eggs.
Together, thermal heterogeneity of microhabitat and behavioral abilities of lizards seems to buffer the negative effects of extreme local heat conditions on developing embryos. Pregnant S. stejnegeri lizards are active mostly on Dsh microhabitat provided by vegetal cover available, because Te on FS and CS microhabitats exceed embryo's thermal tolerated threshold (34 °C) throughout the day. Additionally, these lizards move between different microhabitats, which allow them to be active for several hours for feeding, exploration, and social interactions (Bauwens et al., 1996, Carrascal et al., 1992, Kearney et al., 2009).
Gravid females of oviparous S. horridus have the availability of soil under shade with the physical characteristics (sandy and relatively near to water) described as suitable for nesting for other species belonging to Sceloporus genus (Warner & Andrews, 2002). Shaded places are facilitated by canopy that covers almost 100% of direct sunlight (Parker, Tinoco-Ojanguren, Martínez-Yrízar, & Maass, 2005). The data obtained in artificial/hypothetical nests suggest that these lizards could buffer negative impact of high environmental temperatures on their offspring by simply being active and nesting under the shade provided by vegetal cover (Angilletta, 2009, Doody, 2009, Telemeco et al., 2009), rather than digging deeper nests in full sun where temperatures exceeds the maximum threshold tolerated by S. horridus offspring. Data from artificial/hypothetical nests are a good indicator for the temperatures at which embryos can be exposed because like natural nests, their temperature is regulated by the temperature of the surrounding soil (Brown and Shine, 2004, Campbell and Norman, 1998). Therefore, the behavioral selection of appropriate nesting sites under the canopy could be the most plausible strategy for embryos to avoid extreme heat at the Xalitla locality.
We suggest that the specific behavioral mechanisms described here are the potential strategies that have allowed the 2 studied Sceloporus species to achieve suitable temperature regimes for healthy development of their offspring, and perhaps for the suitable performance of their own physiological processes, in our study localities, which have presented historical and current temperatures above the critical thermal threshold. Therefore, exploiting thermal heterogeneity of microhabitat by behavioral mechanisms, could be crucial to population's persistence in sites with historic and current warmer temperatures (Goller et al., 2014, Sears et al., 2011, Sunday et al., 2014). Furthermore, for future temperature rise scenarios we found that thermal regimes remain similar to the historical and current temperature ranges therefore, behavioral responses could be useful to lizard populations and do not face a threat of erosion caused for a stressful warming in the future; however, it depends on the fact that microhabitat heterogeneity is not modified by human activities (Scheffers et al., 2014, Sunday et al., 2014). Thus, the availability of shaded areas for tropical ectothermic organisms becomes a key resource for them to buffer the impacts of macroclimatic higher temperatures (Huey and Tewksbury, 2009, Kearney, 2013, Kearney et al., 2009, Scheffers et al., 2014).
Here, we provide empiric evidence that lizards, as well as other species are not inert biological entities unable to respond to environmental changes as previous studies suggest (Sinervo et al., 2010). However, there is a need to integrate such information in studies on the effects of climate change on biodiversity for a better understanding of potential species responses to face global warming. Future research should explore other potential ways of organismic adaptation to a rapid environmental warming, such as genetically regulated changes in ranges of physiological tolerances (Leal and Gunderson, 2012, Moritz et al., 2012), biomolecular development of mechanisms of protein production at the cellular level that provide full corporal adaptation to hyperthermia (Evgen’ev, Garbuz, Shilova, & Zatsepina, 2007), phenotypic plasticity in thermal physiology traits (Niehaus, Angilletta, Sears, Franklin, & Wilson, 2012), and to evaluate the rate at which this changes occurs (Huey et al., 2012).
With this in hand, we will have a broader overview to understand the potential effects of global warming on species and their potential responses. Even though, behavioral strategies can be useful to buffer the negative impact of the temperature rise, but could be not enough to cope with a fast extreme warming or unpredictable and longer drought periods and landscape fragmentation. In some cases, behavior could limit local adaptation of thermal physiology required to deal with this phenomenon (Barrows et al., 2010, Buckley et al., 2015, Huey et al., 2003, Sinervo et al., 2010).











 nueva página del texto (beta)
nueva página del texto (beta)


