Introduction
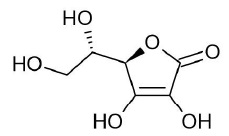
Chili pepper encompasses a variety of wild and cultivated species belonging to the genus Capsicum of the Solanaceae family [1,2]. For many years, chili pepper fruits have been used as an important culinary ingredient with high nutritional value. Additionally, potential applications of chili pepper fruits in traditional medicine for the treatment of various illnesses have been described. Extracts of chili pepper fruit have an effect on lipid metabolism, antimicrobial activity, anticlotting effect, anti-inflammatory and pain-relieving, anticancer, antidiabetic/antihyperglycemic activities, and anticholesteremic, among others [3]. These medicinal uses of chili pepper fruits are due to the accumulation of important amounts of bioactive compounds with valuable nutraceutical and health promoting potential. These phytochemicals include capsaicinoids, carotenoids (provitamin A), ascorbic acid (vitamin C), tocopherol (vitamin E), flavonoids, phenolic compounds, and minerals (calcium and iron, among others) [4-8]. Some of these metabolites are well known for their antioxidant properties, particularly, the ascorbic acid (AsA) constitutes a powerful free-radical scavenger and an effective antioxidant [9]. L-ascorbic acid (L-threo-hex-2-enono-1,4-lactone) is an organic molecule of six carbon atoms derived from sugar; its molecular formula is C6H8O6 (Fig. 1) with a molecular weight of 176.13 [10].
According to Machlin (1992), AsA was discovered in 1912, isolated for the first time in 1928 from adrenal glandules [11], and its structure was elucidated and chemically synthesized in 1933 [12]. Subsequently, a procedure for large-scale preparation of AsA from fruits of a Hungarian pepper (Capsicum annuum) was described in 1934 [13]. Because of the AsA discovery, the Hungarian biochemist Albert Szent-Györgyi was awarded the Nobel Prize of Medicine in 1937.
It has been proposed that ascorbic acid (AsA) is capable of reducing the damage caused by diverse free radicals, usually produced by biological systems, and diminishing the reduction of molecular oxygen to superoxide; chemically, this happens because of the donation of a single hydrogen atom via protonation reactions and separate electron transfer [14]. AsA is an essential molecule for cellular metabolism; nevertheless, anthropoid primates, guinea pigs, teleost fishes, some bats, and Passeriformes birds are not capable of synthesizing this important molecule, because mutations in the gene encoding the L-gulono-γ-lactone oxidase (GLO) enzyme, which catalyzes the last step of the biosynthesis pathway of vitamin C, are responsible for this failure [15]; therefore, they must obtain it from fresh vegetables and fruits, representing chili pepper fruits a grateful source of AsA. Evaluation of thirteen fruits, eight legumes and three tubers consumed in the Andean regions, revealed that chili pepper ají ratón fruits (C. chinense Jacq.) contain higher concentrations of AsA in comparison with fruits such as “guayaba”, “naranjilla” or “pepino dulce” [16]. Table 1 summarizes the content of vitamin C in some fruits.
Table 1 Content of vitamin C in some fruits.
| Fruit | Ascorbic acid content (mg/100 g) | Reference |
| Chili pepper fruits | 11.9-195.8 | [17] |
| Kiwifruit, cultivar Golg | 91 | [18] |
| Papaya cultivar Sunrise | 77.1 | [18] |
| Guava | 65.8 | [18] |
| Pineaple cultivar Sweet Gold | 61 | [18] |
| Orange | 58.3 | [19] |
| Kiwifruit, cultivar Hayward | 55.2 | [18] |
| Grapefruit | 49.15 | [19] |
| Lemon | 43.96 | [19] |
| Mango cultivar Palmer | 40.9 | [18] |
In plants, AsA is an important regulator of different biological processes and a potent antioxidant; for example, AsA is involved in plant growth and development, participates in cell division, cell wall metabolism and cell expansion, shoot apical meristem formation, root development, photosynthesis, regulation of flowering, and regulation of leaf senescence. Additionally, AsA is an essential cofactor for enzyme activity and contributes to the plant’s antioxidant capacity and heavy metal evacuation and detoxification. Finally, AsA plays a crucial role in stress defense in plants including the attack by pathogens [20].
For humans, the consumption of vitamin C is fundamental for the maintenance of good health; vitamin C-deficient diets cause scurvy disease [21]. The increase of AsA concentration in human cells, promotes a significant decrease of oxidative stress and inflammation, having important health benefits, and even small increases in the uptake of vitamin C cause a positive effect on the oxidative and inflammatory status of blood cells [22]. AsA is an important immunomodulatory (impacts on the innate and adaptative immune system), it possesses antimicrobial, antiparasitic, antifungal, antibacterial, and antiviral properties, and represents a novel option for the treatment of infections produced by multidrug-resistant bacteria [23]. Vitamin C has emerged as an excellent candidate for the prevention and treatment of several human diseases and, of course, for promoting good health (Table 2).
Table 2 Medical applications of vitamin C.
| Application | Mechanism of action | Reference |
| Antioxidant | Preventing the oxidative events in organelle membranes of red blood cells and liver. | [24] |
| Analgesic | Antinociceptive effect and postoperative pain relief. | [25] |
| Anticancer agent | Hydrogen peroxide-induced oxidative stress and DNA demethylation mediated by translocation enzyme activation. | [26] |
| Anticancer activity | Redox mechanisms and co-factor activity for 2-oxoglutarate-dependent dioxygenases. | [27] |
| Prevention and treatment of cancer | Stimulating the production and activation of immune cells. | [28] |
| Prevention and treatment of COVID-19 | In synergy with quercetin, shows antiviral and immunomodulatory action. Capacity of AsA to recycle quercetin. | [29] |
| Manage Psychiatric Disorders | Neuromodulator in the central nervous system. | [30] |
| Skin health | Neutralizing free radicals, capable of interacting with hydroxyl, superoxide, and free oxygen ions. Photoprotection, wound healing, anti-pigmentary. | [31] |
| Prevention of ill effects induced by radiation | Mitigating radiation-induced damage. | [32] |
In this review, we summarized the current knowledge about the AsA biosynthesis, its accumulation, and the effects of agricultural practices and chili pepper fruit postharvest processing.
Biosynthesis of ascorbic acid in chili pepper fruits
Although AsA is a ubiquitous molecule in eukaryotes, the series of reactions generating the aldono-1,4-lactone, the immediate precursor of AsA during the biosynthetic pathway, differs between mammals, plants and green algae, fungi, and photosynthetic protists [33]. Biochemical and molecular analyses have demonstrated that the AsA biosynthetic enzyme L-gulonolactone oxidase (GULO) was replaced by L-galactonolactone dehydrogenase (GLDH) in photosynthetic eukaryote lineages following plastid acquisition during the eukaryote evolution [34]. In plants, particularly, different AsA biosynthetic pathways with distinct precursor molecules have been described: L-galactose pathway [35], D-galacturonic acid pathway [36], myo-inositol pathway [37], and L-gulose pathway [38]. From them, the L-galactose pathway has been considered the predominant pathway in diverse fruits [39]. In this sense, the role of the L-galactose, D-galacturonic and myo-inositol biosynthetic pathways of AsA were evaluated using labeled precursors: L-[14C1]-galactose, [3H(G)]-D-galacturonic acid and [14C(U)]-myo-inositol, in Serrano chili pepper fruits (Capsicum annuum cv. Tampiqueño 74) as the study model [40]. Results revealed higher incorporation of labeled L-galactose into AsA, compared with the other labeled molecules; additionally, higher accumulation of transcripts of genes encoding the biosynthetic enzymes of the L-galactose pathway in comparison with levels of transcripts of genes encoding enzymes of the other pathways (myo-inositol and L-galacturonic acid pathways) were reported [40]. Taken together, these results suggest a predominant role of L-galactose pathway in the biosynthesis of AsA in chili pepper fruits (Fig. 2) [40].
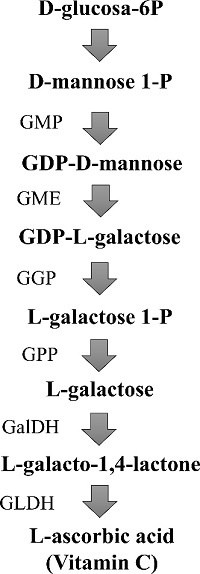
Fig. 2 Ascorbic acid biosynthetic pathway in chili pepper (L-galactose pathway). GMP: GDP-D-mannose pyrophosphorylase; GME: GDP-D-mannose-3´,5´-epimerase; GGP: GDP-L-galactose phosphorylase; GPP: L-galactose-1-phosphate phosphatase; GalDH: L-galactose-1-dehydrogenase; GLDH: L-galactono-1,4-lactone dehydrogenase (Modified from [40]).
L-galactose pathway consists of a series of eight successive reactions that ends with the intermediary L-galactone-1,4-lactone, which is converted directly to AsA by the action of L-galactano-1,4-lactone dehydrogenase (GLDH) (Fig. 2). GLDH has been found in the inner membrane of the mitochondria; other AsA biosynthetic enzymes are located in the cytosol, indicating that most reactions of AsA biosynthetic pathway occurs in the cytoplasm of plant cells [35, 39]. Because of the importance of the GLDH in the biosynthesis of AsA, this enzyme was biochemically characterized, and the GLDH gene was cloned and sequenced from chili pepper fruits; this information is important for designing future biotechnological alternatives to increase AsA content in these fruits. [41]
Regarding the regulation of AsA biosynthetic pathway, it has been reported that in plants, this process is modulated at the transcriptional and post-transcriptional level [42]. In Arabidopsis thaliana, the Ascorbic acid Mannose pathway Regulator 1 (AMR1) has been described as a modulator of the expression of genes encoding AsA biosynthetic enzymes of the L-galactose pathway in response to environmental factors [43]. In the same way, the transcription factor AtERF98 has been proposed as an important regulator of the biosynthesis of AsA in A. thaliana [44]. Nevertheless, these or other regulators of AsA biosynthetic pathway have not been evaluated in chili pepper fruits. In that regard, coexpression analysis carried out between the expression profile of MYB transcription factors and the expression profile of GLDH gene during the ripening process of chili pepper fruits (C. annuum cv. Tampiqueño 74), revealed a positive correlation between CaMYB16 and GLDH expression profile, suggesting that CaMYB16 might regulate the expression of GLDH [45].
On the other hand, it has been observed that AsA accumulation in chili pepper fruits results from the biosynthesis, recycling, and degradation pathways; a negative correlation between AsA concentration and the expression of biosynthetic genes suggests a feedback mechanism that regulates the homeostasis of AsA in these fruits. Additionally, the ascorbate oxidase (AOX) enzyme, which is involved in the degradation of AsA, plays a critical role in the regulation of AsA content during chili pepper fruit development and ripening [46]. Moreover, an increase in the ascorbate content of chili pepper fruits was observed when they were exposed to a nitric acid (NO)-enriched atmosphere; according to that, an increase of about 40 % in the GLDH gene expression and enzymatic activity was recorded. These results suggest that NO constitutes a potential modulator of AsA content in chili pepper fruits [41].
Finally, it has been found that the accumulation of AsA in diverse varieties of chili pepper was correlated with the levels of expression of the AsA biosynthetic genes in a gene-metabolite relation [47].
Ascorbic acid contents in chili pepper fruits
As mentioned in the introduction of this review, chili pepper represents an important source of nutriments and high variation in the content of these bioactive compounds associated with the species, variety, colour, and geographic location has been reported. An analysis of the content of nutrients in over 100 different types of chili peppers native to the Americas, revealed that the AsA content in 90 types analyzed ranged between 11.9 to 195.8 mg per 100 grams fresh weight of chili pepper, and 43 % of fruits exhibited a content of vitamin C higher than the value recommended by the U.S. Food and Drug Administration (60 mg). Additionally, 16 types of the evaluated chili peppers had amounts of vitamin C higher than kiwi, a fruit that is well known for its high AsA content; from them, ‘Trinidad 7 Pot’ chili pepper showed the highest vitamin C content (195.8 mg per 100 g), and interestingly, it was extremely pungent [17]. In the same way, a study carried out with 63 accessions of C. annuum from the Balkan region, showed that the genotype with the highest content of vitamin C (“CAPS-7”) also corresponded to the most pungent accession [48]. Moreover, analysis of 261 landraces and cultivated varieties of C. chinense showed a range of vitamin C content between 30 to 1,466 mg/100 g fresh weight, and it was suggested that this variability was associated with the biosynthesis and metabolism of the compounds related to the flavour of fruits [49]. A metabolomic analysis in mirasol chili pepper fruits (C. annuum) infected with Candidatus Phytoplasma trifolii, revealed an important decrease in AsA concentration, capsaicin, and dihydrocapsaicin in the infected fruits, corroborating the correlation between the accumulation of vitamin C and pungency [50], and suggesting a positive correlation between pungency (content of capsaicin) and AsA accumulation [51].
Variations in the content of AsA associated with the species of chili pepper have been also reported; nevertheless, an investigation conducted with different accessions of C. annuum, C. frutescens, C. chinense and C. baccatum with evident morphological differences (pungency, colour, and origin) showed that all accessions accumulated concentrations of vitamin C up to 200 mg/100 g fresh weight independently of its phenotype [52]. Regarding the accumulation of vitamin C in different parts of chili pepper fruits, higher concentrations of AsA in the pericarp, lower amounts in the placenta, and zero content of vitamin C in seeds have been recorded [53-55]. Finally, a negative correlation between the morphometric characteristics of chili pepper fruits and the content of bioactive compounds has been observed, suggesting that large-sized fruits could present lower concentrations of vitamin C [56]. The relation between the colour of the pericarp of chili pepper fruits and the accumulation of vitamin C has been investigated; it has been described that purple, black, and white fruits exhibited lower levels of AsA in comparison with green, yellow, red, orange, and brown fruits [57,58].
A determinant factor for the accumulation of bioactive compounds is the ripening process; the content of metabolites such as capsaicinoids, organic acids, ascorbic acid, citric acid, malic acid, and phenolics, among others, increase during the maturation of chili pepper fruits [59-61]. These increases in the amounts of nutrients have been associated to physicochemical changes in colour transitions, the improvement of flavour quality, and increased antioxidant activity [62,63]. Specifically, it has been extensively reported that chili pepper fruits accumulate high levels of vitamin C during ripening [64-67]. Although the first stages of maturation process correspond to an increase in fruit size, a gradual accumulation of AsA during the green-to-red transition and a subsequent diminution in the last stages of maturation (red partially and red fully fruits) were reported for seventeen cultivars of C. annuum and one cultivar of C. frutescens [68]. In Serrano ‘Tampiqueño 74’ chili pepper fruits, an increment in the concentration of AsA was reported at 20 days after anthesis (DAA), reaching its maximum at 40 DAA and remaining constant at 50 and 60 DAA [40]. On the other hand, sweet pepper (C. annuum) showed an increment in the concentration during the transition from green to orange fruit, correlating with the conversion of chloroplasts into chromoplasts, the loss of chlorophyll and the biosynthesis of carotenoids [46]. According to that, it has been reported that the content of AsA in the symplast and apoplast increased during the ripening process of C. annuum, and consequently, the activity of AOX decreased [69]. Finally, at the late stages of maturation, when the total antioxidant activity has reached its maximum level, the content of vitamin C and phenolic compounds decreased [70].
On the other hand, it has been described that environmental conditions (such as whether), and harvesting practices (production season) have an effect on the accumulation of bioactive compounds in chili pepper fruits [71,72], but in some cases, as occur in bell chili peppers (C. annuum cv. California Wonder and Excalibur), cultivated by certified organic versus conventional practices, no differences in the accumulation of antioxidants associated to cropping systems or environmental influence were reported [73]. The addition of amendments to native soil (yard waste, sewage sludge, chicken manure, and not much bare soil), showed that differences in the accumulation of AsA were associated with the state of maturation of the fruit, but not to the treatments; nevertheless, the content of other bioactive compounds associated to the addition of amendments was described [74]. Other reports suggest that organic growing systems caused an increase in the level of antioxidants such as vitamin C in sweet bell chili pepper [75]. On the other hand, Lo Scalzo and collaborators evaluated two genotypes of sweet pepper grown for three years in distinct types of cultivation: two organic and one conventional. They concluded that the local cultivar selected for organic production showed higher levels of AsA and sugars [76]. This evidence suggests that in addition to the growing conditions, the accumulation of antioxidants could depend on the genotype (variety and/or accession of chili pepper) [75,77]. However, a high effect of genotypic variation has been associated with sugars and flavonoids, while concentrations of AsA, organic acids, and isoprenoids have been associated with a variable degree of genotypic and stage of growth and ripening of sweet chili pepper varieties [78]. In this sense, grafting has been used as an agronomic technique to improve the yield, water stress tolerance, quality of fruits, and antioxidant systems of chili pepper [79, 80]; studies conducted in two varieties of bell pepper, showed significant differences in bioactive compounds such as AsA, between grafted and non-grafted varieties; therefore, grafting seems to improve the nutritional quality of chili pepper fruits [81].
The effect of modified light quality on the production of bioactive compounds in chili pepper fruits was tested. Experiments conducted with eleven cultivars of sweet pepper, which were cultivated in a plastic tunnel and white shade net under controlled temperature, showed that temperature increased the content of AsA in most cultivars [82]. Additionally, red, and yellow sweet peppers grown under pearl nets, showed higher amounts of vitamin C; thus, the use of selective pearl nets could be an interesting option to improve the nutritional quality of chili pepper fruits [83]. Finally, the addition of pre-harvest compounds such as nitrophenolates in a foliar spray or in the irrigation system increased the accumulation of antioxidants such as AsA in chili pepper plants (C. annuum L. cv. Lamuyo) [84]. On the other hand, the use of WM13-24 biofertilizer supplemented with Bacillus sp. strain Ydj3 as bioagent promoted a significant increase in the ascorbic acid content [85].
Post-harvest storage seems to influence the nutritional quality of chili pepper fruits. Kasampalis and collaborators carried out an analysis of variance, and they described that the nutritional composition of bell chili peppers was affected in the first instance by the ripening stage and harvest process, and to a lesser extent by the period of postharvest storage [86]. It has been suggested that the temperature of storage is important to minimize the losses of vitamin C in chili pepper fruits; in fact, the content of AsA in green mature and chili peppers at the breaking colour stage stored at room temperature increased during the first ten days, equaling the levels found in red chili peppers in plants; however, stored red chili peppers showed a decrease of 25 % in the levels of vitamin C. Finally, it has been reported that refrigeration maintains constant the concentration of AsA for up to 20 days [87]. On the other hand, a decrease in the levels of vitamin C after two days of storage was reported, and a gradual reduction of AsA was observed in fresh chili peppers stored at 5, -20, and -40 °C as compared with freeze-dried chili peppers stored at a range of temperature of -60 °C to -40 °C, but a gradual diminution of AsA was also observed [88]. Other experiments that evaluated different temperatures of storage (4, 25, and 50 °C), during intervals of 30 days, concluded that the content of vitamin C decreased as the time and temperature progressed. Additionally, by covering chili pepper fruits with β-ciclodextrins (β-CD), a loss of 10 % in the concentration of vitamin C in comparison with the control was recorded after 30 days at 50 °C [89]. With the aim of increasing the post-harvest storage-life of C. annuum L., castor oil at temperature of 4-1 °C, has been used as an external coat of chili pepper fruits; this protective coat was efficient during 36 days in comparison with fruits without it (18 days); nevertheless, a significant diminution of vitamin C levels was reported during storage [90].
Another strategy tested for the maintenance of nutritional quality of chili pepper fruits was the implementation of photo selective nets (pearl, red, yellow, or black colour) during the post-harvest storage; fruits under pearl colour nets showed higher levels of vitamin C. It has been suggested that red/far red photon ratio under pearl net could improve the content of AsA in fruits during the post-harvest storage [91]. Gamma radiation of chili pepper fruits has been evaluated to reduce losses of vitamin C during storage; a reduction of 5-10 % of AsA was detected with the increase of the radiation dose. During storage up to 4 weeks, losses dependent of the temperature were detected, but the losses of vitamin C in irradiated fruits were lower than those in non-irradiated controls [92]. Additionally, gamma radiation was used for irradiation of dried hot peppers packed in polyethylene bags. This treatment did not affect the initial levels of bioactive compounds, and the concentration of vitamin C decreased only 12-14 % during the storage for 90 days at room temperature [93]. Finally, low-cost bags with perforations (0-4) were used for packing Jalapeño chili peppers stored at 7 °C for 6 weeks, and the results showed that the levels of vitamin C remained unchanged, representing a good option for the preservation of bioactive compounds in chili peppers [94].
Effects of processing on the ascorbic acid content
Chili pepper fruits are commercialized both fresh and dried; it has been reported variations in the content of vitamin C in these fruits. Analysis of the content of AsA in fresh and dried fruits of C. chinense, C. annuum (var. abbreviatum, acuminatum, and grossum), and C. frutescens (var. bacatum) demonstrated a decrease of about 10 % in the concentration of AsA, being the fresh fruits those that accumulated higher amounts of vitamin C [67,95-97]; some other authors reported a decrease up to 88 % in the content of AsA in drying chili peppers, but freezing previously to drying reduced significantly the loss of vitamin C [87].
The effect of different drying methods in the accumulation of AsA has been described. Kumar and coworkers used freezing, microwave vacuum, sun drying, and hot air as drying methods for five cultivars of C. annuum var. annuum; they concluded that microwave vacuum drying was the most efficient method to minimize the loss of nutritional quality of chili pepper fruits [98]. Other approaches have used a forced air oven or a solar dryer and compared with lyophilized and commercial chili peppers; nevertheless, the methods implemented caused a significant reduction in the content of vitamin C in fruits of chili peppers [99]. Although pre-drying methods, such as centrifugation, have resulted in a marked loss of vitamin C in chili pepper fruits [71], pretreatments before drying such as blanching, potassium metabisulfite (KMS) dip, different concentrations of citric acid (CA) dip and combinations of them, have been tested in fruits of sweet bell pepper. Samples treated with KMS and CA and dried in solar poly tunnel showed higher amounts of AsA in comparison with the other pretreatments [100]. High-humidity hot air impingement blanching used as pretreatment before drying the red peppers was implemented; fruits were stored for six months at ambient temperature. The results showed a reduction of 33-59 % in the concentration of vitamin C, suggesting that blanching pretreatment could minimize the loss of AsA in fruits stored for several months [101]. On the other hand, the use of blanching in acetic acid solution and soaking immediately with a solution of Na2S2O5 and CaCl2, were tested as a pretreatment; samples consisting in green chili paste, green chili longitudinal slit, whole red chili, and untreated green chili paste were prepared and dried. In this case, the pretreatment reduced the time of drying and preserved the amount of bioactive compounds in the samples analyzed, including the content of AsA [96].
Additional to drying methods, the fruits of Capsicum are subjected to other processing methods for its commercialization. It has been described that processed (pickled and chipotle canned) Jalapeño and Serrano chili peppers, contained lower concentration of bioactive compounds and lower antioxidant powder in comparison with fresh fruits; a decrease of 12 % during water blanching, and 20-25 % in canning process were recorded. Nevertheless, freezing is capable of increasing the retention of AsA up to 60 % and this percentage increased up to 87 % when chili pepper fruits were previously blanched [102]. Moreover, thermal processing of Jalapeño fruits, caused a 75 % diminution of vitamin C content [64]. Some other approaches have been tested including the effect of thermal (blanching in water and steam) and non-thermal (sonication, pulsed electric field and their combinations) on the content of bioactive compounds of red bell pepper. Chili peppers subjected to thermal treatments showed significant reduction in bioactive compounds, vitamin C, among others. On the other hand, non-thermal processing of chili peppers provoked similar or slightly reduction in the content of bioactive compounds compared with untreated fruits [103].
Finally, the process of cooking could affect the antioxidant properties of chili peppers; previous reports have quantified the loss of AsA during preparation and cooking up to 26.1 % [104]. Pickling (acidification with vinegar) is a common procedure for the preparation of chili peppers; it has been reported that the content of vitamin C of green hot peppers decreased during the first three weeks of storage after pickled processing and did not change after eight weeks. Interestingly, the addition of sugar and chickpea in pickling chili peppers, increased up to 48.2 % the retention of AsA in three weeks, and 40.9 % in eight weeks of storage [105]. On the other hand, it has been described that radical-scavenging activity in pickled chili pepper (C. annuum L.) was maintained during the first 28 days of storage at 35 °C, but then, a decrease of 47 % was observed [106].
Other cooking methods such as boiling, steaming, stir-frying and roosting, and 5, 10, and 15 minutes used as cooking time were implemented, and then the retention of AsA in chili pepper fruits was evaluated. The content of vitamin C was reduced from 24.3 % to 66.5 % in boiling and steaming process, whereas stir-frying and roasting cooking process provoked a reduction of 2.7 % to 25.9 % [107]. Finally, using sauteing as cooking method of chili pepper fruits, did not cause a significant reduction in the content of AsA [17].
Conclusions
Because vitamin C represents an important bioactive compound with notable medical application, most of the works has focused on the improvement of its content with different agricultural practices and the evaluation of the effects of processing and storage in the accumulation and integrity of AsA in chili pepper fruits. Nevertheless, some molecular mechanisms such as the transcriptional regulation of the vitamin C biosynthetic pathway or the possible epigenetic regulation are still poorly understood, representing a great opportunity for future investigations.











 nueva página del texto (beta)
nueva página del texto (beta)