Introduction
Some strategies to prevent obesity are control food intake or alter lipids metabolism by inhibiting fat absorption [1]. Pancreatic lipase (triacylglycerol acylhydrolase) is the most important enzyme in the digestion of 50-70 % of the fat diet and, it is responsible for the hydrolysis of triacylglycerols (triglycerides) to monoacylglycerols and free fatty acids [2].
Tetrahydrilipstatin (Orlistat TM), a derivative of lipstatin obtained from Streptomyces toxytricini, is the only pancreatic lipase inhibitor currently approved for the treatment of long-term obesity. Its use can result in up to 10 % weight loss when used in combination with diet and physical activity. However, this drug can cause adverse liver effects (cholelithiasis, colostatichepatitis, and sub-acute liver failure) and troublesome gastrointestinal symptoms [3]. The inhibition of pancreatic lipase in many plants has been studied for its anti-obesogenic potential. In addition, the use of natural products to control obesity increased the interest in finding inhibitors of pancreatic lipase without the orlistat side effects [4].
Many medicinal, herbal, and edible plant extracts have been reported with in vitro inhibitory activity against pancreatic lipase, mainly in plants of eastern countries e.g., Malaysia, China, Jordan, and Korea [5,6,7]. One of the studies with the highest number of plants (400 plants) searching to inhibit pancreatic lipase activity was reported by Roh and Jung [8]. Mexico is a country with a high biodiversity of plants. However, there are only two studies focused on searching for a plant with a high inhibitory activity of pancreatic lipase. Ramirez et al. [9] reported that among 23 medicinal plants used in traditional medicine for diabetes, only two plants had 31.4 % and 27.2 % of lipase inhibitory activity. The second study [10] reported four plants with pancreatic lipase inhibition ≥ 60 % by analyzing a suite of 30 medicinal plants from Oaxaca State. This study aims to analyze 37 ethanol extracts from different parts of plants, easy to obtain, to assess their anti-lipase activity.
Experimental
Plant materials
Table 1 shows the plants and the parts tested. Some plants are edible, medicinal, or belong to a family that has the inhibitory activity of pancreatic lipase. The parts of the plants used in this study were based on the lack of studies in these tissues. The compound’s composition changes with the use of fresh or dry material and for this reason, both were studied [11,12]. Some species were obtained from a local supermarket and others were acquired from a local greenhouse in Puruándiro, Michoacán, Mexico. The plant identity was confirmed by the taxonomist Patricia Silva of the Department of Botany of the University of Michoacán, and a voucher specimen was deposited in the herbarium of the University of Michoacán (EBUM). The voucher number of Hibiscus rosa-sinensis is EBUM240774.
Table 1 Inhibitory concentration (IC50) of pancreatic lipase activity of the plant extracts.
| Scientific Name | Family | Part Used | IC50 (µg/mL) |
| FCucumis sativus | FCurcubitaceae | FP | >400 |
| FHeliopsis longipes | Asteraceae | Rt | >400 |
| Nelumbo nucifera | Nelumbonaceae | FL | >400 |
| Rubus sp. | Rosaseae | FR | >400 |
| Salvia hispánica | Lamiaceae | FL | >400 |
| Solanum melongena | Solanaceae | FR | >400 |
| Tamarindus indica | Fabaceae | FR | >400 |
| Vaccinium corymbosum | Ericaceae | FR | >400 |
| Vitis sp. | Vitaceae | FL | >400 |
| Amorphophallus konjac | Araceae | FR > | >400 |
| Fragaria vesca. | Rosaseae | S | >400 |
| Heidichium coronarium | Zingiberaceae | FR > | >400 |
| Hibiscus rosa-sinensis | Malvaceae | FL | >400 |
| Lavandula | Lamiaceae | DL | >400 |
| Opuntia sp. | Cactaceae | C | >400 |
| Pisttia stratiotes | Araceae | DL | >400 |
| Tigridia pavonia | Iridaceae | B | >400 |
| Vitis sp. | Vitaceae | DL | >400 |
| Annona muricata | Annonaceae | FL | 324.37 |
| Callistemon citrinus | Myrtaceae | DL | 383.46 |
| Cucumis melo | Curcubitaeae | Se | 235.44 |
| Morus sp. | Moraceae | FL | 249.06 |
| Morus sp. | Moraceae | DL | 391.06 |
| Salvia hispánica | Lamiaceae | Se | 334.06 |
| Spinacia oleracea | Amaranthaceae | DL | 219.83 |
| Syzygium jambos | Myrtaceae | FL | 262.19 |
| Amorphophallus konjac | Araceae | DRh | 218.21 |
| Annana comosus | Annanaceae | FR | 39.53 |
| Byrsonima crassifolia | Malpighiaceae | FR | 163.93 |
| Capsicum sp. | Solanaceae | FL | 278.03 |
| Carica papaya | Caricaceae | Se | 44.63 |
| Fragaria sp. | Rosaseae | FR | 45.35 |
| Hibiscus rosa-sinensis | Malvaceae | DL | 73.82 |
| Hibiscus sabdariffa var. rubra | Malvaceae | FL | 44.73 |
| Lavandula | Lamiaceae | FL | 3 |
| Passiflora sp. | Passifloraceae | FL | 121.93 |
| Syzygium jambos | Myrtaceae | DL | 207.27 |
Plant parts: FL: Fresh Leaves, Se: Seeds, DL: Dry Leaves, FR: Fruits, Rt: Roots, P: Peel, FRh: Fresh Rhizome, DRh: Dry Rhizome, S: Sepal of a flower, C: Cladode, B: Bulb. The results are the mean ± SD (n = 4).
Plant extractions
Leaves, fruits, seeds, roots, peel, Opuntia sp. cladode, strawberry sepal, and rhizomes were washed with water. Some leaves were dried in the convection oven at a temperature of 40 °C for two weeks. Dried and fresh samples were grounded into fine powder. Three grams of the sample were placed in 30 mL of 98 % ethanol for seven days at room temperature. The solvent was evaporated under 45 °C and low pressure. The dried extracts were dissolved in 1 % of dimethyl sulfoxide (DMSO).
Pancreatic lipase inhibition
The plant extracts obtained at 1 mg/mL were diluted in 1 % DMSO at 50, 100, 200, 300, and 400 μg/mL to perform an inhibition curve. 0.2 mL of the different concentrations were added to 0.1 mL of a pancreatic lipase solution (1 mg/mL); then, Tris-HCl buffer pH 7.4 was added to up to 1 mL and incubated for 15 min at 37 °C. After that, 0.1 mL of p-nitrophenyl-butyrate (100 mM) was added and incubated for 30 min at 37 oC; then, the samples were read at 405 nm. Orlistat was used as a reference control [13]. IC50 is the concentration that gives 50 % lipase inhibition.
Antioxidant activity determinations Radical-scavenging activity (RSA) assay
Free radical scavenging capacity was analyzed by the 2, 2-diphenyl-1 picryl hydrazyl (DPPH) assay according to the method of Hatano et al. [14]. Trolox was used as standard in a range of 25-800 µM.
Ferric reducing antioxidant power (FRAP) assay
The FRAP assay was performed using the method described by Thaipong et al. [15].
Trolox equivalent antioxidant capacity (TEAC)
The assay was performed as Rufino et al. [16] with modifications that reduced the time to obtain the ABTS.+ radical cations to two hours. It was produced by mixing an equal volume of 2,2´-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) liquid substrate system (ready-to-use) trade Sigma, and 2.4 mM potassium persulfate. The mixture was shaken for 2 h at room temperature in the dark. At this time the absorbance reached a value of 3.0; the ABTS.+ solution was diluted with ethanol to get an absorbance of 0.70 ± 0.02 at 734 nm. Fresh ABTS.+ solution was prepared for each assay. A sample (0.05 mL) was added to ABTS solution (0.95 mL) after mixing, and the reaction was monitored for 7 minutes. Trolox standard was in the range of 25 - 600 µM. TEAC values were expressed as Trolox equivalents (TE)/g fresh mass.
Total phenolic content
The amount of the total phenolic was determined with the Folin-Ciocalteu reagent using the method of Pripdeevech et al. [17]. Gallic acid was in the range (0.01-0.4 mM). The results were expressed as mg of gallic acid equivalent (GAE)/g fresh mass.
Total flavonoid content
The amount of the total flavonoid was determined using the method of Chang et al. [18]. Rutin acid was used to calculate the standard curve (0.025 to 0.5 mg/mL).
Animals and experimental design
Male Wistar rats from 8-12 weeks old (200-300 g), were housed at a room temperature of 24 °C, with a 12 h light/dark cycle, fed with chow standard diets and water ad libitum. The rats were from the animal house of the Universidad Michoacana de San Nicolás de Hidalgo (UMSNH). The protocol for these experiments was approved by the Institutional Committee for Use of Animals of the UMSNH and followed the recommendations of the regulatory standard for the use of animals issued by the Mexican Ministry of Agriculture in its Federal Regulations for the Use and Care of Animals (NOM062-ZOO-1999). Thirty male Wistar rats that had fasted overnight were divided into five groups: one control group and the rest four groups were administered with oral gavage of 3 mL of a lipid emulsion, (6 mL corn oil, cholic acid 80 mg, 5 mL egg yolk and 6 mL of saline solution), with slight modifications [19]. Groups 2, 3, and 4 were administered orally with dried leaves extract of Hibiscus rosa-sinensis at a concentration of 62.5, 125, and 250 mg/kg respectively, and the 5th group with orlistat (50 mg/kg p.o.), as a positive control. Blood samples were collected from the tail every hour for three hours. Triacylglycerol concentrations were determined using Spinreact Kit.
Results
Pancreatic lipase (PL) inhibition activity
37 plant extracts were tested at the concentrations of 50, 100, 200, 300, and 400 μg/mL against the pancreatic lipase activity. Nine plants had a low percentage of inhibition of lipase activity ˂ 41 %; nine extracts displayed a medium percentage of inhibition (41-50 %); eight extracts presented high inhibition in the range of range 51-60 %, and 11 extracts produced the highest percentage of lipase inhibition, ≥ 61 %. Table 1 shows that some extract plants had IC50 values up to 400 μg/mL. Although this study worked with a few specimens (37 plant extracts), 19 plants showed high inhibitory pancreatic lipase activity which represents 51.3 % of the plants. Moreover, all plant extracts presented inhibition against pancreatic lipase (Fig. 1).
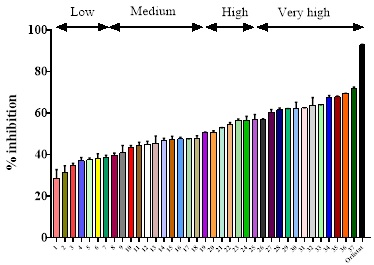
Fig. 1 The inhibitory effect of 37 ethanolic extracts on pancreatic lipase activity is divided into four groups: low (˂41%), medium (41-50 %) high (51-60 %), and very high (≥ 61 %). The values are the mean ± SD (n = 4). 1. Heliopsis longipes; 2. Cucumis sativus; 3. Rubus sp.; 4. Tamarindus indica; 5. Vaccinium corymbosum; 6. Salvia hispánica leaf; 7. Solanum melongena; 8. Vitis vinifera fresh leaf; 9. Nelumbo nucifera; 10. Amorphophallus konjac fresh bulb; 11. Pisttia stratiotes; 12. Tigridia pavonia; 13. Vitis vinifera fresh leaf; 14. Opuntia sp.; 15. Lavandula sp. dry leaf; 16. Hibiscus rosa-sinensis fresh leaf; 17. Fragaria sp. sepal; 18. Heidichium coronarium; 19. Morus sp. dry leaf; 20. Callistemon citrinus; 21. Salvia hispánica seeds; 22. Morus sp. fresh leaf; 23. Syzygium jambos fresh leaf; 24. Annona muricata; 25. Spinacia oleracea; 26. Cucumis melo seeds; 27. Amorphophallus konjac dry bulb; 28. Capsicum sp.; 29. Byrsonima crassifolia; 30. Annana comosus; 31. Lavandula sp. fresh leaf; 32. Hibiscus sabdariffa; 33. Carica papaya; 34. Fragaria sp.; 35. Passiflora sp.; 36. Syzygium jambos dry leaf; 37. Hibiscus rosa-sinensis dry leaf.
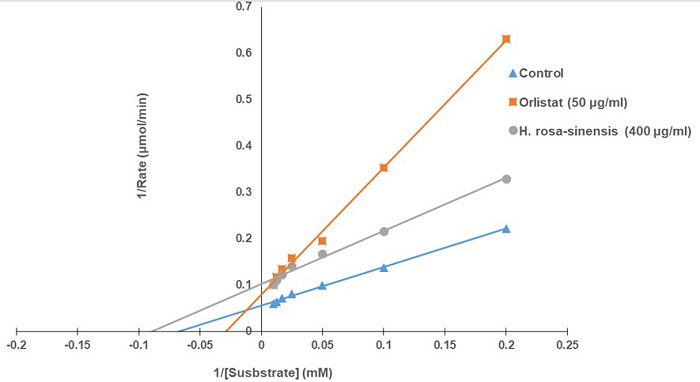
Hibiscus rosa-sinensis (Malvaceae), commonly known as China rose. The dried leaf extract displayed the highest inhibitory activity of pancreatic lipase, 71.90 % at 400 µg/mL, very similar to orlistat 70% at 50 µg/mL. The inhibitory action of H. rosa-sinensis dried leaf extract was dose-dependent in a range of inhibitor concentrations (50-400 µg/mL). The enzyme kinetics of H. rosa-sinensis, using the graphical representation of the Lineweaver-Burk, showed an uncompetitive inhibition with Km of 11.11 μM and Vmax value of 10 μmol/min, whereas Km value of orlistat for pancreatic lipase was of 28.5 μM and the Vmax was 16.6 μmol/min, also showed an uncompetitive inhibition (Fig 2).
Antioxidant activity
Hibiscus rosa-sinensis showed the highest PL inhibitory activity; as a result, this plant was selected to evaluate the antioxidant capacity using the DPPH, FRAP, and ABTS assays. In all assays, H. rosa-sinensis dried leaves had higher antioxidant activity and total phenolic compounds than fresh leaves. Conversely, fresh leaves contained a higher number of flavonoids than dried leaves (Table 2).
Table 2 Antioxidant activity and the total amount of phenolic and flavonoids of ethanol extracts of Hibiscus rosa-sinensis leaves
| Dry | Fresh | |
| DPPH (μM Trolox/mL) | 500.03 ± 1.50 | 356.76 ± 8.08 |
| EC50 DPPH | 293.23 ± 10.57 | 380.40 ± 0.38 |
| FRAP (μM Trolox/mL) | 646.62 ±1.84 | 432.55 ± 1.55 |
| EC50 FRAP | 175 ± 0.98 | 360 ± 0.87 |
| ABTS (μM Trolox/mL) | 443.86 ± 2.64 | 241.66 ± 3.24 |
| Phenolics GAE (mg/g f.w) | 26.91 ± 0.82 | 13.15 ± 0.80 |
| Flavonoids Rutin (mg/g f.w) | 30.33 ± 0.65 | 44.57 ± 1.65 |
DPPH: EC50 (μg/mL) effective concentration at which 50 % of DPPH radicals are scavenged, Trolox 150 μg/mL. FRAP: EC50 (μg/mL) effective concentration at which the absorbance is 0.5, Trolox 75 μg/mL (Values are given as mean ± SD (n = 4).
Oral administration of lipid emulsion in rats
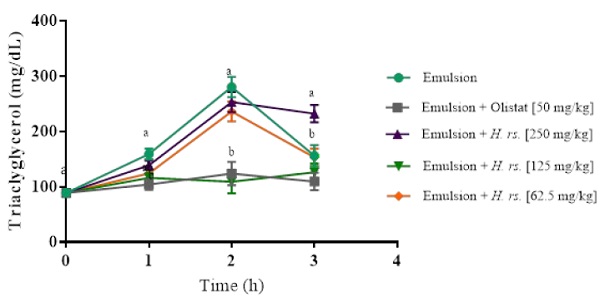
Fig. 3 shows the reduced absorption of lipids effect of H. rosa-sinensis dried leaves in vivo. Plasma triacylglycerol levels were measured after oral administration of lipid emulsion. 125 mg/kg of H. rosa-sinensis significantly reduced the postprandial levels of triacylglycerol, after 2 h of oil emulsion administration, similar to orlistat. Meanwhile, triacylglycerol levels increased in the other groups.
Discussion
This study tested 37 plant extracts, with different uses in Mexico, to evaluate their anti-lipase activity. Pancreatic lipase is the main enzyme responsible for the digestion of dietary triacylglycerols [2]. Therefore, the inhibition of enzymes involved in the assimilation of lipids is an important issue to prevent obesity.
11 plants presented inhibition ≥ 61 %; in this group, dried rhizome and leaves of Amorphophallus konjac, Hibiscus rosa-sinensis, and Syzygium jambos presented higher inhibitory activity of pancreatic lipase, than the leaves in a fresh state. However, the opposite occurred in Lavandula spp., where the fresh leaves had a higher inhibitory activity of pancreatic lipase than dried leaves. These results could be explained by considering that in some species the drying process can alter the phytoconstituents (phenolics and flavonoids) [20,21]. Moreover, as reported by Dewi et al. [22] in Rubus fraxinifolius Hayata, fresh leaves had a higher content of phenolics and flavonoids and antioxidant capacity than dry leaves, but the α-glucosidase inhibitory activity was lower. However, the opposite occurred in dry leaves. Morus sp. did not show a difference in pancreatic lipase inhibition between fresh and dried leaves; both presented a high anti-lipase activity. Some plant components that have been reported for pancreatic lipase inhibitory activity are flavonoids, lignans, polyphenols, terpenes, and saponins [23].
Hibiscus rosa-sinensis dried leaves extract showed the highest pancreatic lipases inhibitor activity, presenting a dose-response curve. It is a glabrous shrub widely cultivated in the tropics and has several forms with varying colors of flowers. One of the first properties of flowers and leaves was hair growth-promoting. Other uses are to treat arterial hypertension, hypoglycemic, laxative, and oral contraceptives [24]. In some Asia countries and even in Mexico, leaves have been used for dysentery, diarrhea, and as analgesic [25]. In Nigeria, the young leaves of H. rosa-sinesis is eaten [26]. Rios-Chavez et al. [27], reported as the major constituents of H. rosa-sinesis leave extract: fructose (18.60 %), linoleic acid (9,12-octadecadienoic acid 18:2 n-6) (10.36 %), α-linolenic acid (9,12,15-octadecatrienoic acid 18:3 n-3, ALA) (7.67 %) and glycerol (6.l8 %). Seeds of H. rosa-sinensis are rich in unsaturated fatty acids and high content of ALA; however, H. rosa-sinesis leaves have an abundant composition of linoleic and α-linolenic acids. These compounds are important as a dietary component.
Linoleic acid and ALA are unsaturated fatty acids that humans cannot synthesize. These fatty acids are beneficial to health, besides being a precursor of arachidonic acid and having antioxidant properties, and anticarcinogenic and antiartherogenic effects [28].
This is the first study reporting the pancreatic lipase inhibitory activity of H rosa-sinensis. In the genus Hibiscus, Buchholz and Melzig [29] showed that the methanol and aqueous extract of H. sabdariffa flowers has an inhibitory activity of pancreatic lipase of 100 % at a concentration of 2500 μg/mL. However, our study showed 70 % of inhibition of lipase activity at a concentration of 400 μg/mL. Moreover, Kumar et al. [30] found a hypolipidemic activity of H rosa-sinensis roots ethanol extract, when used in a high-fat diet in rats.
Fig. 2 shows the inhibition mode of PL by H. rosa-sinensis at 400 μg/mL using a Lineweaver-Burk plot. The kinetic study revealed that H. rosa-sinensis extract decreases the Km and Vmax values, implying that it has uncompetitive inhibition of pancreatic lipase activity. In this type of inhibition, the inhibitor binds to the enzyme-substrate complex, and not to the enzyme, preventing the product formation, and making this mode of action useful for enzyme-target drug design [31].
Most of the studies of the antioxidant capacity in Hibiscus rosa-sinensis do not mention the flower color; this characteristic, as reported by Patel et al. [32] is important. These authors studied the antioxidant properties of five cultivars (red, yellow, orange, pink, and white) of H. rosa-sinensis, finding differences in their DPPH radical scavenging activities. The leaf extract of the red cultivar showed EC50 389.2 µg/mL. This finding was consistent with our results that showed an EC50 380.40 and 293.20 µg/mL in fresh and dried leaf extracts of the red cultivar of H. rosa-sinensis.
There are few reports about the reducing power of H. rosa-sinensis. Patel et al. [32] found that the yellow cultivar presented the highest reducing power at 1000 µg/mL and the lowest reducing power was found in the white cultivar. Divya et al. [33] reported a high ferric reducing power in methanol extract of leaves using 95 µg/mL. Our study showed EC50 values of 360 and 175 µg/mL in fresh and dry leaves of H. rosa-sinesis, respectively. Most of the studies use dry tissues because some conditions as storage, time, and temperature could affect antioxidant activity [14,34]. Conversely, there are reports showing that drying alters the phytoconstituents as phenolic, flavonoids, total antioxidant capacity, and some enzymatic activities [18,19,20].
In this study, the total phenolic and flavonoid contents were similar as reported Ghaffar and El-Elaimy [35]. Moreover, Wong et al. [36] screening six Hibiscus species in Malaysia and found that antioxidant properties change as a function of the environmental conditions. The total phenolic content in the leaves and flowers of H. rosa-sinensis was lower than our results. In addition, a study on the leaves and flowers of Hibiscus roseus presented lower phenolic and flavonoid content. However, phenolic compounds have potential applications in the skin [37].
Our study showed, for the first time, that dried leaves of H. rosa-sinesis presented higher antioxidant capacity, phenolic content, and inhibitory pancreatic lipase activity than fresh leaves and revealed a positive correlation between the dried state and biological activities.
Dietary fat promotes more body fat storage than dietary carbohydrate causing obesity, hyperglycemia, hypertension, and atherosclerosis, pathologies included in the metabolic syndrome [38]. Therefore, plants that possess the capacity to inhibit digestion and absorption of dietary fat are a good alternative to treating obesity. Tetrahydrolipostatin (Orlistat TM) is a pancreatic lipase inhibitor that does not affect the nervous system or enter the bloodstream [39]. However, it has many gastrointestinal side effects [40]. Few works measured plasma triacylglycerol levels after oral administration of a lipid emulsion in rats. As shown in Fig. 3, 125 mg/kg of H. rosea-sinensis leaf extract presented a reduction in triacylglycerol levels, similar to orlistat. Li et al. [41] reported that sesame meal presented pancreatic lipase inhibitory activity and the major compounds were linoleic acid and oleic acid. They also showed that these two fatty acids reduced fat digestion. As mentioned above, linoleic acid is one of the major compounds in H. rosa-sinensis, so its anti-lipase activity may be produced by this compound. The finding that H. rosa-sinensis reduced the elevation of plasma triacylglycerol in a similar way to orlistat did, suggests a relationship with the inhibitory pancreatic lipase activity. H. rosa-sinensis dried leaf extract may be useful for body weight control.
Conclusions
Based on the analysis of 37 plants, H. rosa-sinensis showed the highest inhibition of pancreatic lipase activity that displayed a positive correlation with the antioxidant capacity. Consequently, H. rosa-sinensis can be used to reduce body weight. Further studies are required to identify the active compound that shows inhibitory pancreatic lipase activity.











 nueva página del texto (beta)
nueva página del texto (beta)