Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos de cardiología de México
versión On-line ISSN 1665-1731versión impresa ISSN 1405-9940
Arch. Cardiol. Méx. vol.80 no.2 Ciudad de México abr./jun. 2010
Artículo de revisión
Anatomic diagnosis of congenital heart disease. A practical approach based on the sequentiality principle
Diagnóstico anatómico de las cardiopatías congénitas
Jorge Cervantes–Salazar,1 Pedro Curi–Curi,2 Samuel Ramírez–Marroquín,3 Juan Calderón–Colmenero,4 Luis Muñoz–Castellanos.5
1 Subjefe del Departamento de Cirugía Cardiaca Pediátrica y de Cardiopatías Congénitas.
2 Cirujano Cardiovascular Pediátrico.
3 Jefe del Departamento de Cirugía Cardiaca Pediátrica y de Cardiopatías Congénitas.
4 Subjefe del Departamento de Cardiología Pediátrica.
5 Médico Especialista en Ciencias Morfológicas.
Instituto Nacional de Cardiología Ignacio Chávez
Corresponding author:
Jorge Cervantes Salazar.
Instituto Nacional de Cardiología Ignacio Chávez.
Juan Badiano N° 1, Colonia Sección XVI,
Tlalpan. Mexico City.
Phone: 5554 5828 ext. 1352. Fax: 52 (55) 5573 0994.
E–mail: jlcersa@yahoo.com
Received in September 2, 2009.
Accepted in October 16, 2009.
Abstract
Based on the sequentiality principle, this review proposes a practical method that allows the systematization of the anatomic diagnosis of congenital heart disease. We emphasize the need to use sequential connection between the different cardiac segments: atria, ventricles and great arteries. Five ordered steps are defined, which include determination of atrial situs and of the connection features between the ventricles and the great arteries. Related lesions and some additional special features are a second stage in the sequential analysis of congenital heart disease, which is also important for the integral diagnosis.
Key words: Congenital heart disease, Anatomy; Cardiac connection; Sequential diagnosis; Mexico.
Resumen
En esta revisión se propone, en base al principio de secuencialidad, un método práctico que permite sistematizar el diagnóstico anatómico de las cardiopatías congénitas. Se enfatiza la necesidad de utilizar la conexión secuencial entre los distintos segmentos cardiacos que son los atrios, los ventrículos y las grandes arterias. Se definen cinco pasos ordenados que incluyen, en primera instancia, la determinación del situs atrial y de las características de la conexión entre los ventrículos y las grandes arterias. Las lesiones asociadas y algunas particularidades adicionales ocupan una segunda etapa en el análisis secuencial de las cardiopatías congénitas, que también tienen importancia en el diagnóstico integral.
Palabras clave: Cardiopatía congénita; Anatomía; Conexión cardiaca; Diagnóstico secuencial; México.
Introduction
Anatomically, there are six basic principles for the reconstruction of the human body: antimery, metamery, pachimery, stratigraphy, polarity and sequentiality. Among them, antimery and sequentiality are the most important for the anatomic diagnosis of congenital heart disease (Figure 1). The basis of antimery is the division of the body and/or any of its organs by means of a sagital plane, which results in two halves. These halves –right and left– are called antimers and, as a general rule, they are both morphologically and functionally asymmetric. In the heart, the sagital plane is represented by the septum, and antimers are represented by the right and left cardiac chambers which have different morphological features, which allow them to be differentiated from one another and which are also functionally different.1
On the other hand, sequentiality is based on the description of body segments consecutively connected to each other, regardless of their spatial relationship. Connection refers to the anatomic continuity between two segments, while relationship means the spatial position existing among them.2,3 Based on the sequentiality principle, we distinguish three main anatomic segments in the heart: the atria, the ventricles and the great arteries. These three segments are sequentially connected to one another in both antimers, forming pulmonary and systemic circulation circuits. Venous segments, connected to the atria, must be considered as secondary or additional ones.
The purpose of this review is to propose, on the basis of the above–mentioned anatomic principles, a practical method that allows for the systematization of the diagnosis of congenital heart disease. This method consists on a series of five steps that will be described, and that when followed in order, they will allow for a complete anatomic diagnosis of any kind of congenital heart disease, no matter how complex it may be. The first step consists on defining the atrial situs; the atrio–ventricular connection must be subsequently analyzed, followed by the connection features between the ventricles and the great arteries; then, related defects must be determined, followed by some additional special features.2–4
Atrial situs: In normal conditions there are two anatomically well–differentiated atria in both cardiac antimers that, as it was previously stated, are morphologically and functionally asymmetric. The right atrium has two essential anatomic features: the triangular shape of its appendix, with a wide base incorporated to the atrial cavity showing the inner cresta terminalis and the pecti–neus muscles. On the other hand, the left atrium is also characterized as such due to the shape of its appendix, which shows an elongated configuration in the form of a glove finger with a narrow base, which is not incorporated to the atrial cavity (Figure 2). The right atrium receives the superior caval vein and the inferior caval vein, which seem to pull it longitudinally, thus giving it an elongated appearance. Conversely, the left atrium receives the four pulmonary veins that seem to pull it transversally, thus giving it a widened appearance.

There are four types of atrial situs: two asymmetric types (situs solitus and situs inversus), and two symmetric types (dextroisomerism and levoisomerism). In cases of atrial situssolitus, the right atrium is in anterior and right position and the left atrium is in a backward and left position. In the two cases of situs inversus the mirror image of situs solitus is observed. Both in dextroisomerism and levoisomerism, atrial cavities have morphologically similar appendixes. In dextroisomerism there are two atria with right atrium anatomic features and in levoisomerism both atria have left atrium anatomic features. In 2001, the Machado–Atías et al,5 Venezuelan Group proposed a new terminology for symmetrical atrial situs: dextromorphism and levomorphism, to be used instead of dextro and levoisomerism, respectively. We agree with this terminology, as we consider it to be more appropriate for the anatomic diagnosis, and we believe that the terms dextro and levoisomerism should be applied in physical and chemical definitions.
Highly significant contributions have been made to atrial situs diagnosis in the last decades. In the past, it was determined by the electrocardiogram P–wave morphology and by the position of the liver's right lobe. When P wave was positive in DI and negative in aVR it was said to be a case of situs solitus, whereas in cases of situs in–versus diagnosis resulted from P wave negative in DI and positive in aVR. On the other hand, when the liver's right lobe was on the right situs solitus was diagnosed, whereas when the liver was placed to the left, situs inver–sus was diagnosed. This rule is adequate for most simple heart disease cases but there are a lot of exceptions to it in complex ones. Literature review shows that in more than 50% of cases with complex malformations, there is a discrepancy between visceral and atrial situs.4,6 In such circumstances, a more constant relationship was sought between visceral and atrial situs. With rare exceptions, there is a very constant relationship between bronchial and atrial anatomy.7,8 That's why a thoracic x–ray or a bronchial tomography is extremely useful in the diagnosis of atrial situs. In situs solitus cases, the bronchium located to the right shows the right–type anatomic features, which are a smaller length, straight line direction and proximal bifurcation, whereas the left bronchium has a bigger length, curvilinear direction in superior concavity and distal bifurcation. In dextroisomerism cases, we find two bronchi with similar right–type features, whereas in levoisomerism there are two bronchi with left–like type features. In situs solitus cases, the relationship between the left and right bronchi's extension ranges from 1.5 to 2.0. In other words, the left bronchium is one and a half times or twice longer than the right one.
If there are doubts as to the atrial situs type, we may clarify them by means of echocardiography or ultimately by means of an angiocardiogram in the atria. In cases of situs solitus, dimensional echocardiography shows the inferior caval vein to the right side of the aorta. This relationship is inverted in cases of atrial situs inversus. In the right isomerism, the aorta and the inferior caval vein are on the same side of the spine, with the aorta behind the inferior caval vein, whereas in levoisomerism we observe the interruption of inferior caval vein and the systemic venous return reaches the heart by means of azygos veins, located on the same side of the spinal cord together with the aorta in backwards position.9,10 Using the same procedure, the morphology of appendixes may be identified and atrial situs may be diagnosed through paraesternal and subcostal approximations.
Atrio–ventricular connection: Ventricles are recognized by the trabecular septum's anatomic features. The right ventricle (Figure 3) has a trabecular septum with very thick muscular bands and it also has a structure, the moderator band, inserted in the interventricular septum and in the free wall of the right ventricle, whereas the left ventricle has a trabecular septum with very thin muscular bands in the apical section and it is smooth in the upper section (Figure 4). The former has a group of papillary muscles inserted in the free wall, and the latter has two groups of papillary muscles which are also inserted in the free ventricular wall. Trabecules provide a cavernous–type inner morphological appearance to the right ventricle, whereas the left ventricle has an areolar appearance.


The atrio–ventricular connection is biventricular when each atrium is connected to a ventricle, and it is univentricular when both atria are connected to a single ventricle. In the atrio–ventricular connection, the type and the mode of junction between the atria and the ventricles must be analyzed. The atrio–ventricular connection type refers to the anatomy of the atrio–ventricular junction. There are five types of atrio–ventricular connection: concordant, discordant, ambiguous, double ventricular inlet and absent connection. In the concordant atrio–ventricular connection, the right atrium is connected to the right ventricle and the left atrium is connected to the left ventricle; in the discordant atrio–ventricular connection, the right atrium is connected to the left ventricle and the left atrium is connected to the right ventricle, regardless of the spatial position that these two segments have with respect to each other. This way, we may have a concordant atrio–ventricular connection with the right atrium to the right and with the right ventricle to the left, and a left atrium to the left with a left ventricle to the right. This form of spatial relationship is known as criss–cross or crossed atrio–ventricular connections.11 For the diagnosis of concordant or discordant atrio–ventricular connections it is necessary to have a lateralized atrial situs. The atrio–ventricular connection is said to be ambiguous when the atrial situs is isomeric and each atrium is connected to one ventricle. There is a double inlet to a ventricle when most of the atrio–ventricular annulus diameter or more than 75% of a common atrio–ventricular valve is connected to one of the ventricles. Finally, there is absence of an atrio–ventricular connection in the type of connection that lacks continuity between the atrium and the ventricle on the same heart's an timer, with an absence of the corresponding atrio–ventricular valve. The case of absent right connection would be the classic tricuspid atresia, and the case of absent left connection would be mitral atresia. In case of tricuspid atresia, the right atrium is not connected to the ventricular mass. It is separated from it by the deep atrio–ventricular sulcus and most of it rests on the left ventricular mass. The right atrium actually communicates with the left atrium by means of an interatrial septal defect and it potentially communicates with the ventricular mass by means of the subinfundibular septal defect.
The atrio–ventricular connection mode refers to the connection form existing between the atria and the ventricles. This takes place through two permeable atrio–ventricular valves, through a perforated and an non–perforated valve, through atrio–ventricular valves on horseback on the interventricular septum, or through a common atrio–ventricular valve.4,12 In the case of a non–perforated valve, it is important to emphasize that exists a true valvular atresia, unlike those with a lack of an atrio–ventricular valve, which are typically called tricuspid atresia or mitral atresia. With regard to the on horseback atrio–ventricular valves, these are so considered when a 50% of the valve annulus diameter is riding on each ventricle. In this case they are assigned to their respective ventricles, whether the connection is concordant, discordant or ambiguous. If the riding exceeds 50% on the non–corresponding ventricle, the atrio–ventricular connection is considered as a double ventricular inlet, either right or left, in accordance with the ventricle involved. If there is 75% "riding" the atrio–ventricular connection type may be concordant, discordant or ambiguous and it if exceeds 75% on one ventricle, it is considered as a double ventricular inlet.4
Ventricular–arterial connection: Similarly, the ventricular–arterial connection must be analyzed strictly in function of its anatomic features. This method will avoid terminological confusions in some complex malformations with significant distortions at the arterial level, and which due to the fact that they have not been analyzed in relation with their anatomic features they have encouraged the use of different nomenclatures for the same malformation, such as clinical transposition, malposition of the great arteries, isolated ventricular inversion, transposition with posterior aorta, among others.3,13
There are four types of ventricular–arterial connections: concordant, discordant, double outlet and single outlet connections. It is said that the ventricular–arterial connection is concordant when the pulmonary artery is connected to the right ventricle and the aorta is connected to the left ventricle. In the discordant connection, the aorta is connected to the right ventricle and the pulmonary artery is connected to the left ventricle. It is said that there is a double outlet of the right or the left ventricle when one annulus is fully connected, and more than 50% of the other is connected to one of the ventricles, or when more than half of the two sigmoid valve annulus are connected to a ventricular chamber. We will exceptionally come across cases in which both sigmoid valve annulus are on horseback on the interventricular septum in a 50% of their diameter. In order to define the terms, we would say that in these cases there is a double outlet of the two ventricles. Logically, ventricles may be well shaped or they may be a rudimentary chamber. There is a single outlet of a ventricle when only one arterial trunk is connected to the ventricular mass. This way, the truncus arteriosus communis and pulmonary atresia with an isolated aortic trunk (which is the most severe form of tetralogy of Fallot), are the only ways described as a single outlet.13 In the ventricular–arterial connections there may be two perforated sigmoid valves, one non–perforated valve, one or both riding on the interventricular septum, or there may be a single common sigmoid valve connected to the heart. These anatomic features refer to the ventricular–arterial connection mode.
Related lesions: At this stage in the anatomic diagnosis of congenital heart disease, we will describe the related lesions, such as valvular dysfunctions, stenosis or atresia of a valve or an artery, hypoplasia, interruption or communication at any heart level. On Table 1 we propose a classification of the types of related lesions, according to the cardiac segment involved, following the anatomic sequentiality principle, in order to describe the most common heart and great arteries' congenital malformations. These lesions may appear isolated or combined as in tetralogy of Fallot.

Additional special features: In the additional special features we include the position of the heart inside the thorax, and the origin and distribution of coronary arteries, as well as the layout of the conduction system.
The position of the heart inside the thorax is the spatial relationship existing between this organ and the cage inside of which it is placed. The normal position is called levocardia, in which the cardiac apex is located to the left and the cardiac axis goes from the right to the left, from top to bottom, and from back to front. Any variation of this normal pattern is called cardiac malposition, the most frequent ones being dextrocardia, mesocardia and ectopia cordis. Dextrocardia is characterized by the apex to the right, the cardiac axis from the left to the right, and by the presence of most of the cardiac mass in the right hemithorax. There is mesocardia when the heart's apex and major axis occupy a central position in the thorax. Finally, ectopia cordis is a malposition in which the heart is partially or totally out of the thorax, and there may be a cervical, thoracic and thoracic–abdominal form. On the other hand, it is important to underline that regardless of the position that the heart adopts in the thorax, ventricles may have a different topography.
Anatomically, there are some practical rules related to ventricular topography: 1) the left ventricle is backwards in relation with the right ventricle when there is a coherence between its situation and that of the cardiac apex; example: a left ventricle situated to the left with the apex to the left or a left ventricle situated to the right with the cardiac apex to the right; 2) the left ventricle is in anterior position with respect to the right ventricle when there is a discrepancy between the position of that left ventricle and the position of the heart's apex; example: left ventricle to the right with cardiac apex to the right or left ventricle to the right, with cardiac apex to the left; 3) both ventricles are situated on the same frontal plane when the heart as a whole, is situated in the center of the thorax and its apex does not point to the left or to the right but simply forward; 4) when there is an inferior and a superior ventricle (ventricle in two floors), it has always been observed that the inferior one has left–type morphology and the superior one has right–type morphology. These rules are valid when ventricles are of similar size and they are altered when there is hypoplasia aggravated in one of the cavities, and it may also be modified due to the existence of hemodynamic alterations.13
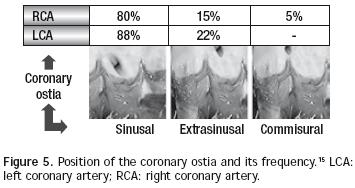
There are normally two coronary arteries: the right one (generally dominant, as it originates 70% of times the posterior descending artery) and the left one (which originates the anterior descending and circumflex arteries).14 Both arteries come from independent coronary ostia. According to the implantation height in relation with an imaginary line that joins the aortic sigmoid valves' junctions, coronary ostia location may be designed as sinusal, extra–sinusal or commissural. Habitually, the left coronary ostium is placed in a sinusal location in a higher percentage than the right ostium (88% vs 80%), as the latter is the only one that may be located commissuraly (5%). The extra–sinusal location is also more common in the left ostium (22%) than in the right one (15%) and commissural location of the left ostium is exceptional.15 Figure 5 illustrates these concepts and exposes frequency percentages of each of these types according to our studies.16 Coronary arteries layout may be very variable, but the most common pattern (68%) is originated from the left coronary trunk that starts at the level of the anterior valve and it is divided into anterior descendent and circumflex arteries, with the right coronary artery arising at the level of the left posterior valve. The second most common pattern (20%) is that of two independent coronary ostia, but with the circumflex artery arising from the right coronary artery. Other less common patterns are: single right coronary ostium (4.5%), inverted coronary arteries (3%), intramural coronary arteries (2%), and single left coronary ostium (1.5%).17 It is also worth stating that the main pathological variations of the coronary arteries are anomalous left coronary artery from the pulmonary artery (ALCAPA syndrome), and the coronary fistulae.
Cardiac conduction system is a set of myocardial fibers specialized in the dronotropic function, which normally includes five components: the sinus node (or Keith and Flack node, located in the superior cavoatrialjunction), the internodal fascicles (anterior or Bachmann's fascicle, medium or Wenckebach's fascicle and posterior or Thorell's fascicle), the atrio–ventricular node (or Aschoff Tawara's node, located in the Koch's triangle, limited by Todaro's tendon, the tricuspid annulus and the coronary sinus' ostium),15 the bundle of His (with its right branch – descending along the septomarginal trabecula – its left branch, and the latter's anterior and posterior subdivision), and Purkinge's net. The presence of pectineus muscles not only allows for the morphological identification of a right or a left atrium, but it is also useful as a guide to establish the sinus node's location. According to European studies,3,18 in atrial dextromorphisms (asplenia syndome), the sinus node is bilateral and symmetrical and is closely related to the superior cavoatrial junction, whereas in atrial levomorphisms it is extremely hypoplasic and it is located far away from the cavoatrialjunction. In cases of atrial isomorphism and ambiguous connection with the normally lateralized ventricles, the atrio–ventricular node is located in a mirror image of Koch's triangle, whereas in atrial isomorphisms with ambiguous atrio–ventricular connection and inverted ventricles it has a more anterior location and it is even surrounding any related interventricular defect. On the other hand, His' bundle always passes by the interventricular septum that is a limit to both ventricles (even if any of them is hypoplasic). Therefore, the interventricular sepum is the one carrying the cardiac conduction axis, regardless of the atrio–ventricular connection and the arterial ventricle, and regardless of a biventricular or univentricular anatomy (right–type or left–type morphology). This way, the adequate identification of the atrio–ventricular junction and of the morphology of a single dominant ventricle, allows us to establish the location of the atrio–ventricular node and of the cardiac conduction axis.18
In conclusion, it may be said that anatomic diagnosis of congenital heart disease is the best procedure for understanding a congenital cardiac malformation from the anatomical and physiopathological point of view. The relationship between the different segments must be analyzed once its anatomy is known, but it lacks physio–pathological significance. Finally, it is worth stressing that the most important clinical application of the sequential anatomic diagnosis of congenital heart disease is determined by the connection and by the type of related lesions, which are the ones that will provide the steps to be followed when choosing a therapeutic alternative.
References
1. O'Rahilly R. Anatomía de Gardner. 5th Edition. Ed. Interamericana McGraw Hill. (México D.F. México) 1989; 3–9. [ Links ]
2. Van Praagh R. Segmental approach to diagnosis in congenital heart disease. Birth Defects 1972; 8:4–23. [ Links ]
3. Shinebourne EA, Macartney FJ, Anderson RH. Sequential chamber localization. Logical approach to diagnosis in congenital heart disease. Br Heart J 1976; 38:327–340. [ Links ]
4. Attié F, Zabal C, Buendía A. Cardiología Pediátrica. Diagnóstico y Tratamiento. Editorial Médica Panamericana S.A. (México D.F. México) 1993:15–19. [ Links ]
5. Machado I, Anselmi G, Machado I, et al. Discordances between the different types of atrial arrangement and the positions of the thoraco–abdominal organs. Cardiol Young 2001;11:543–550. [ Links ]
6. Liberthson RR, Hastreiter AR, Sinha S, et al. Levocardia with visceral heterotaxy–isolated levocardia. Am Heart J 1973;85:40–54. [ Links ]
7. Caruso G, Becker AE: How to determine the atrial situs. Considerations initiated by three cases of absent spleen with discordant anatomy between bronqui and atria. Br Heart J 1979;41:559–567. [ Links ]
8. Rylaarsdam MR, Attié F, Buendía A, et al. Discordancia entre la anatomía bronquial y el situs atrial. Arch Inst Cardiol Méx 1990;60:393–399. [ Links ]
9. Higgins CB, Silverman NK, Kersting–Sommer–Hoff BA, et al. Congenital Heart Disease. Echocardiography and Magnetic Resonance lmaging. New York Raven Press, 1990:315–333. [ Links ]
10. Attie F, Muñoz L, Ovseyevitz J, et al. Crossed atrio–ventricular connections. Am Heart J 1980; 99: 163–172. [ Links ]
11. Díaz G, Attie F, Quero M, et al. Secuencia diagnóstica de las cardiopatías congénitas. Arch Inst Cardiol Méx 1982;52:59–61. [ Links ]
12. Attie F: Cardiopatías Congénitas. Morfología, cuadro clínico y diagnóstico. México. Salvat Mexicana de Ediciones SA. 1985:33-44. [ Links ]
13. Becker AE, Anderson RH. Cardiac Pathology. London. Churchill Livingstone, 1982:9–10. [ Links ]
14. Kouchoukos N, Blackstone E, Doty D, et al. Kirklin/Barrat–Boyes Cardiac Surgery. Third Edition. Vol 2. Ed. Elsevier Science. (New York. USA) 2003;1438–1507. [ Links ]
15. Curi–Curi P, Carvajal H, Tejerina H, et al. Determinación del patrón anatómico valvular, altura y ubicación de los ostia coronarios en corazones de fetos humanos. Cuad Hosp Clin 1994;40(1):28–32. [ Links ]
16. Castañeda A, Jonas R, Mayer J, et al. Cardiac surgery of the neonate and infant. Ed. WB Saunders Company. (St Louis. USA) 1994:409–438. [ Links ]
17. Ho SY, Seo JW, Brown NA, et al. Morphology of the sinus node in human and mouse hearts with isomerism of the atrial appendages. Br Heart J 1995;74:437–442. [ Links ]
18. Anderson R: How should we optimally describe complex congenitally malformed hearts? Ann Thorac Surg 1996; 62(3):710–716. [ Links ]














