Introduction
Domesticated species have been exposed during their evolution to artificial selection that determine the genetic structure, phenotypic patterns and divergence among cultivated and wild populations (Charlesworth et al., 1997; Pickersgill, 2007). Also, artificial selection operates in the domestication processes and has encouraged morphological, physiological, and genetic modifications that could alter mating systems and genetic structure in domesticated organisms (Renaut & Rieseberg, 2015). Some of the consequences of domestication, include loss of genetic diversity, introduction of these genes associated with domestication process, an accumulation of deleterious variants, phenotype convergence and local adaptation to plantations and could represent a profound impact on genomes (Meyer & Purugganan, 2013; Larson et al., 2014).
Particularly, an important crop species in Mexico, the species C. annuum L. (Solanacae) is known as chile and represented one of the most important crop, with a great economic significance (Pickersgill, 2007). The domestication process of C. annuum was conducted in Mexico, due to the morphological variation of varieties (López-Espinosa et al., 2018). Chile represents, one of the ancestral crops of Mexico, where domestication process began between 5000-7000-BC, and occurred at the Tehuacán Valley (Meyer & Purugganan, 2013). Most of local varieties, have been cultivated for long periods with an intermediate cultivation and an agricultural management system (González et al., 2011; Guzman et al., 2019). Regarding their economic importance, several studies evaluated the importance of local varieties of chile using morphological, cytogenetic and genetic markers (Contreras-Toledo et al., 2011). These studies shown, that chile is one of the primary crop in America and Mexico and that different species were domesticated in several provinces independently (Ibiza et al., 2012). Nevertheless, further studies are needed to estimate heterozygosity levels to implement conservation programs for the rational use of the remaining diversity of wild populations (Rodríguez et al., 2007; Guzman et al., 2019).
Oaxaca state, is considered as a region with a high diversity of species (Ortiz-Pérez et al., 2004), as well as many local domesticated species (Contreras-Toledo et al., 2011). Oaxaca possess a high biological and cultural diversity, that coupled with a complex environmental heterogeneity, results in their high biodiversity, represented by more than 12 500 species of flora and fauna; many of which have been utilized by local populations (Contreras-Toledo et al., 2011). An example, of native chilies domesticated at the Oaxaca state, 'Tusta', 'Tabaquero', 'Solterito', 'Piquín', 'Nanchita', 'Costeño' and 'Chile de Agua' (Alonso et al., 2008; Vera-Guzmán et al., 2011). That represented a different degree of domestication, artificial selection and genetic differentiation.
Taking into account the economic importance of this chile resource, is necessary to evaluate their genetic structure to generate procedures for management and conservation. The main objectives of this study are to examine genetic diversity levels of populations of C. annum with different levels of human management located in the valleys and mountains in Oaxaca, Mexico. We addressed the following questions: 1) We characterized, the population genetic diversity, differentiation and genetic inbreeding levels, to test if genetic diversity parameters could be affected by the level of disturbance. (2) We evaluated, the possible occurrence of recent bottlenecks events, estimated the effective population size and the niche similarity and divergence. To understand whether populations undergone recent bottleneck processes and to recognize if populations located in different valleys and mountains significantly differentiated from each other.
Materials and methods
Study system
C. annuum plants are recognized for their bright coloured, fleshy, podlike fruit. Plants are bushy and low growing 30-38cm tall. The stems are to some extent woody with thin, green leaves 4-10cm long and 1-4cm wide (González et al., 2011). White flowers are produced from leaf axils in summer, but are inconspicuous. The fruit usually remains ornamental for 8 to 12 weeks. This species has been organized into five botanical groups of which only three cherry, cone and cluster peppers are familiar potted plants (Pickersgill, 2007). Botanical classification of Capsicum has been difficult, due to the high number of varieties, the deficiency of well-defined traits and the presence of hybridization identified of some species (Ibiza et al., 2012). Chile represented, one of the ancestral and utilized crops of Mexico and domestication occurred at the Tehuacán Valley. Most of the local crops, have been cultivated and consumed for long time with a stable cultivated under an agricultural management system that constitute an important genetic resource (Guzman et al., 2019).
Collecting sites
The study area was designated among two biogeographic regions at the Oaxaca State in Mexico. Young leaves were collected, five from wild populations, five from homegarden and three from domesticated populations at the Oaxaca State (Table 1, Fig. 2). We collected 13 populations distributed in Oaxaca and sampled between 12 - 20 individuals. We sampled from trees separated with at least 1000 m. For sampling, we choose the criteria of geographic location. Genomic DNA was extracted from 100 mg of fresh leaf material using the protocol designed by Lefort & Douglas, (1999). Seven nuclear microsatellite loci, were amplified using the loci previously reported for C. annuum (Minamiyama et al., 2006) and utilizing multiplex polymerase chain reaction approach. PCR techniques were arranged into three different groups. The first group include the primer pairs Agi021, Agi069, Agi098 and Agi111. The second group comprise the primers CAMS163, CAMS460 and CAMS679 (Minamiyama et al., 2006). PCR techniques were performed using the QIAGEN Multiplex PCR kit (QIAGEN) in a 5 µl volume containing 1X Multiplex PCR Master Mix, 2 µM of primer, dH2O, and 20 ng of template DNA. The thermal cycling conditions consisted of 35 cycles of 94°C for 1 min, annealing 51°C for 1 min, extension at 72°C for 2 min and final extension at 72°C for 10 min. Multiplex PCR products were combined with a GeneScan-500 LIZ size standard and performed on an ABI-PRISM 3100 Avant sequencer (Applied Biosystems). Fragments were analyzed and recorded using the Peak Scanner program 1.0 (Applied Biosystems).
Table 1 Locality name, sample size, geographical coordinates, mean number of alleles (A), mean observed heterozygosity (H O ), mean expected heterozygosity (H E ), for 13 populations of C. annum in the Oaxaca, Mexico, separated by wild, homegarden and cultivated populations. Standard errors are included in parenthesis.
| Locality | Sample size | Genetic diversity | ||||
|---|---|---|---|---|---|---|
| Coordinates | A | H O | H E | F IS | ||
| Wild populations | ||||||
| 1. El Coyul1 | 11 | 15.413/ -97.420 | 3.71 (2.07) | 0.700 (0.10) | 0.556 (0.08) | -0.208 (0.05) |
| 2. Zaachila1 | 7 | 18.619/ -97.548 | 5.00 (2.78) | 0.707 (0.24) | 0.671 (0.17) | 0.004 (0.07) |
| 3. Proterill | 10 | 18.403/ -97.548 | 6.14 (2.53) | 0.728 (0.11) | 0.716 (0.14) | 0.035 (0.01) |
| 4. Guleagui | 13 | 18.703/ -97.603 | 4.28 (3.03) | 0.698 (0.12) | 0.589 (0.10) | 0.128(0.05) |
| 5. MorroM2 | 11 | 18.555/ -97.635 | 4.14 (2.08) | 0.589 (0.08) | 0.568 (0.07) | 0.029(0.07) |
| Total | 4.654 | 0.684 | 0.620 | -0.0024 | ||
| Homegarden | ||||||
| 6. Agua de Sol1 | 11 | 18.865/ -97.702 | 4.71 (2.31) | 0.771 (0.16) | 0.576 (0.10) | -0.290 (0.12) |
| 7. Agua de Sol2 | 11 | 18.464/ -97.568 | 4.42 (2.66) | 0.696 (0.08) | 0.560 (0.08) | 0.279 (0.06) |
| 8. Zaachila2 | 7 | 18.409/ -97.429 | 4.28 (0.98) | 0.833 (0.16) | 0.658 (0.10) | 0.170 (0.09) |
| 9. MorroM3 | 10 | 18.358/ -97.480 | 3.57 (2.07) | 0.757 (0.10) | 0.532 (0.07) | -0.178 (0.11) |
| 10. El Coyul2 | 11 | 18.476/ -97.572 | 4.57 (1.21) | 0.650 (0.10) | 0.536 (0.03) | -0.154 (0.01) |
| Total | 4.31 | 0.741 | 0.572 | -0.0346 | ||
| Cultivated | ||||||
| 11. Taviche1 | 10 | 18.619/ -97.477 | 4.71 (2.40) | 0.620 (0.12) | 0.665 (0.07) | 0.121 (0.08) |
| 12. Taviche2 | 11 | 18.605/ -97.542 | 5.14 (1.94) | 0.767 (0.21) | 0.698 (0.10) | -0.032 (0.04) |
| 13. Taviche3 | 11 | 18.636/ -97.422 | 5.57 (1.37) | 0.712 (0.14) | 0.639 (0.03) | -0.060(0.14) |
| Total | 5.14 | 0.699 | 0.667 | 0.0096 | ||
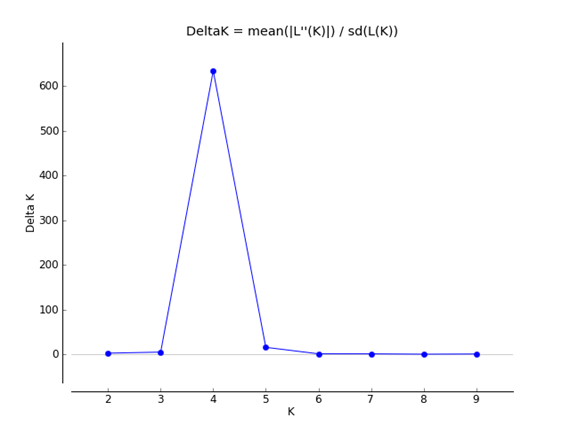
Fig. 1 Values of ΔK plotted against K, the peak indicates the most probable number of genetic groups given the data using Structure Harvester (Earl, 2011).
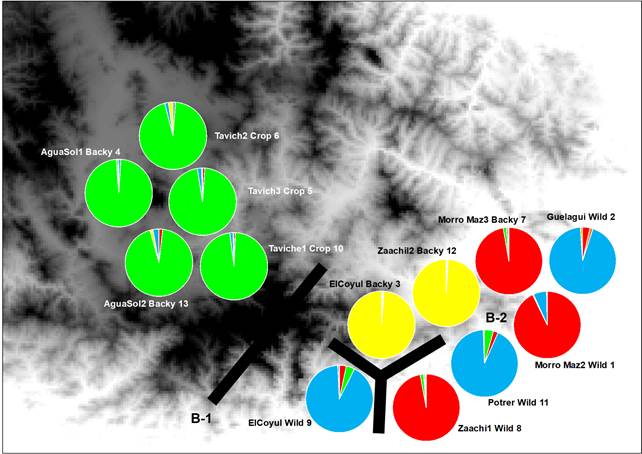
Fig. 2 Each pie chart represents the proportions in each population of the four genetic groups as assigned by the program STRUCTURE. Green, red, yellow and blue Genotype are representing the genetic ancestry groups corresponding to C. annum populations. Genetic discontinuities (bold lines B-1-2) obtained with Monmonier’s maximum difference algorithm on genetic distances derived from microsatellite allele frequencies.
Genetic diversity analysis
For each C. annum populations, the following genetic diversity parameters: number of alleles per locus (A), observed heterozygosity (H O ), expected heterozygosity (H E ) and inbreeding coefficient (F IS ), using GENETIX 4.02 software (Belkhir et al., 1996).
Genetic structure and Bayesian admixture analysis
To test genetic structure, we performed a hierarchical test of population structure (AMOVA), employing the stepwise mutation model (SMM) using ARLEQUIN 3.5. program (Excoffier, & Lischer, 2010). We grouped, C. annum populations taking into account the domestication degree: wild, homegarden and cultivated populations that represented a different extent of domestication, artificial selection and genetic differentiation. Statistical significance was tested using 104 permutations using ARLEQUIN program. We estimated the gene flow between C. annum populations was considered by estimating the Bayesian-scaled long-term effective population size (Ne) and migration rate (m) employing the MIGRATE 3.5.1 software (Beerli & Felsenstein, 2001). For the analyses, the starting chain value was set to 206 visited and 16 recorded genealogies, following a burn-in period of 503 iterations.
The genetic ancestry of each individual plant of C. annum was analyzed with the STRUCTURE 2.3.4 software (Pritchard et al., 2000; Falush et al., 2003; Hubisz et al., 2009). STRUCTURE uses a Bayesian clustering model to determine the proportion of each individual’s ancestry originating from different populations (Evanno et al., 2005). The optimal number (K) of groups was determined by varying K from 1 to 10 (e.g. to achieve the effective number of groups) and running the analysis ten times for each K value to find the maximum posterior likelihood [LnP (D)]. Each run was performed using 106 Markov chain Monte Carlo (MCMC) repetitions following a burn-in period of 504 iterations. We used an admixture model that allows the correlation of allele frequencies without any a priori information. Following the procedure of Evanno et al. (2005), we determined the most likely value of K based on the maximum value of ΔK.
We utilized, two different approaches to achieve genetic clustering, with the programs STRUCTURE and ADEGENET, to contrast the differences among genetic groups. We utilized the Discriminant Analysis of Principal Components (DAPC) using the adegenet 2.0 (Jombart et al., 2010) package in R Version 3.5.1. We implemented the ‘find.clusters’ function to achieve the discriminant analysis of principal component procedure (Jombart et al., 2010). To avoid overfitting, 1000 principal components (e.g. to accomplish the correct number of clusters) were retained, accounting for ∼70% of the total variance. This analysis runs successive K-means clustering and the optimal number of K was selected based on the lowest associated Bayesian Information Criteria (BIC) value after examining the rate of decrease in BIC. We tested values of genetic groups K = 1 - 20 (e.g. to achieve correctly the number of genetic groups) with 10 replicates of each K. The DAPC function was executed using this clustering, retaining 2 axes of principal components analysis (PCA) sufficient to explain 80% of the total variance of the data (Jombart et al., 2010).
To identify geographic barriers and genetic discontinuity among C. annum populations, we used the Monmonier’s maximum difference algorithm with BARRIER v. 2.2 software (Manni et al., 2004). BARRIER creates a map of the sampling localities from their geographical coordinates. Barriers are then represented on the map by identifying the maximum values within the population-pairwise genetic distance matrix. We used a pairwise matrix of average square distances (ASD) estimated for C. annum populations. Resampling random subsets of individuals within populations estimated with the MSA program with 100 bootstrap replicate distances that were used to achieve statistical significance for the predicted barriers.
Testing for niche divergence and conservatism
To determine the ecological niche differentiation between genetic groups C. annum and identify the suitable habitat between genetic groups, we performed niche divergence test, niche overlap, range overlap and niche similarity test using 11 climate variables and using Schoener’s D and Hellinger’s I niche similarity metrics, implemented in ENMtools 1.4.4 software (Warren et al., 2008) and the package ecospat (Broennimann et al., 2012). These indexes quantify the niche similarity, which ranges from 0 (no overlap in habitat suitability) to 1 (identical niche models in habitat suitability). We applied a threshold in which habitat is considered suitable, using the average of between genetic groups “Minimum training Presence logistic” threshold (=0.20). Niches were considered different if the observed value of niche overlap was less than the niche overlap value from 95 or all 100 of the niche overlap values. Niche breadth was calculated as proportional similarity between the observed distribution of the environmental variables (Warren et al., 2008).
To analyze and visualize the ENMs of C. annum, we quantified the niche overlapping, niche equivalence (e.g. assess whether the ENMs between genetic groups are different than expected even if they share the same underlying distribution) and niche similarity (e.g. inquires whether one species niche can predict the occurrence of the other), estimated between genetic groups. For each C. annum genetic groups a PCA-Environmental approach derived from multivariate theory implemented in the package ecospat (Broennimann et al., 2012). The procedure involves a principal component analysis (PCA) to associate climatic values with species occurrence densities in the current period (Warren et al., 2008). All analyses were conducted using R program V. 3.2.3 and ecospat package (Broennimann et al., 2012).
Changes in population size
We used the BOTTLENECK 1.2 software (Piry et al., 1999) to detect population bottlenecks, using the domestication degree: wild, homegarden and cultivated populations of C. annum. Recent population bottlenecks that could be defined as a population where the rare alleles are the first to be lost decreasing the mean number of alleles per locus. In contrast, heterozygosity is less affected, producing a transient heterozygosity excess relative to that expected based on the resulting number of alleles (Luikart & Cornuet, 1998). For this test, we used 90% stepwise and 10% multistep mutations and performed 104 iterations, employing the Wilcoxon’s signed-rank test, the stepwise mutation (SMM), the infinite allele (IAM) and the two-phase mutation (TPM) models. To contrast, the differences among the different mutation models. We estimated the effective population size (Ne) of all locations, using the LDNe software (Waples & Do, 2008). This program implemented the bias-correction technique developed by Waples & Do, (2008) to obtain Ne from a sample of S individuals. We set Pcrit = 0.02 (i.e., alleles with a frequency ˂ 0.02 are excluded), which generally provides a good balance between accuracy and bias. Confidence intervals (CIs) for Ne were calculated with the chi-square approximation implemented in LDNe.
Results
Genetic diversity analysis
Values of genetic diversity parameters for C. annum ranging from high for homegarden locations (A = 4.28-4.71, H O = 0.699-0.833) followed by moderate-high for wild locations (A = 3.71-6.14, H O = 0.589-0.728) and finally lower values for cultivated locations (A = 4.71-5.57, H O = 0.620-0.767) see Table 1. Wright’s inbreeding coefficient within populations (F IS ) revealed in general, positive values representing a heterozygote “reduction” (Table 1); F IS values were low for wild populations (F IS = 0.014-0.244), low in homegarden populations (F IS = 0.170-0. 279) and cultivated populations (F IS = 0.032-0. 121) all values are significant see Table 1.
Genetic structure and Bayesian admixture analysis
Results from hierarchical analysis of molecular variance (AMOVA) separated by domestication degree of C. annum (Table 2), Indicated that most of the genetic variation resided within populations (75.95%), while the differentiation among groups only accounted for the remaining variation (22.76%). Followed by variation among populations within groups (1.28%), all levels are significant (Table 2). Genetic exchange results detected with MIGRATE (Table 3, Fig. 2) outlined that in agreement with genetic structuring, we detected less exchange among populations across long distance such the Cluster 2 (green), (i.e. Structure Analysis) have low exchange with respect to all populations. The rest of the populations that belong to Cluster 1 (blue), 3 (yellow) and 4 (red) show clear indications of genetic exchange values ranging from low to moderated values of exchange among the population and despite belonging to wild, homegarden and homegarden populations (Table 3, Fig. 2).
Table 2 Analysis of molecular variance (AMOVA) performed on the nSSR data and using RST. We grouped, the populations taking into account the domestication degree: wild, homegarden and cultivated populations that represented a different extent of domestication and genetic differentiation. Asterisks indicate statistically significant values (P < 0.01). Tests were based on 104 random permutations.
| Source of variation | SS | Variance components |
Percentage of variation |
Fixation index | |
|---|---|---|---|---|---|
| R ST | |||||
| Among groups | 2 | 0.037 | 1.28 | ΦCT = 0.090*** | |
| Among populations within groups |
10 | 0.664 | 22.76 | ΦSC = 0.010*** | |
| Within populations | 223 | 2.218 | 75.95 | ΦST = 0.080*** | |
| Total | 235.5 | 2.92104 | |||
Table 3 Levels of genetic exchange estimated with the program MIGRATE. Directional pairwise migration rates among eleven C. annum populations from Oaxaca Mexico. Donating and receiving populations are below and above the diagonal, respectively. Migration rates are given as the value of effective number of migrants per generation (Nm). Bold number indicate statistically significant values (P < 0.01).
| Nm | MM2S1 | GuelS2 | Coy1T3 | AgIT4 | Tav3C5 | Tav2C6 | MM3T7 | ZacS8 | Coy2Si9 | Tav1C10 | PotrS11 | Za2T12 | Ag2T13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MM2S1 | ---- | 0.145 | 0.423 | 1.034 | 0.065 | 1.065 | 3.432 | 3.055 | 1.845 | 0.525 | 0.460 | 0.188 | 0.756 |
| GuelS2 | 0.705 | ---- | 0.398 | 1.791 | 1.099 | 5.860 | 0.787 | 1.094 | 1.589 | 1.733 | 1.736 | 0.099 | 9.047 |
| Coy1T3 | 0.225 | 0.246 | ---- | 1.970 | 0.062 | 0.185 | 0.345 | 0.308 | 0.303 | 0.123 | 1.233 | 1.585 | 0.789 |
| AgIT4 | 2.195 | 2.191 | 1.788 | --- | 1.676 | 1.806 | 1.366 | 1.378 | 1.254 | 2.162 | 2.193 | 0.448 | 2.070 |
| Tav3C5 | 1.519 | 1.826 | 1.456 | 1.819 | ----- | 2.952 | 1.833 | 1.190 | 1.846 | 2.271 | 2.575 | 1.592 | 2.138 |
| Tav2C6 | 2.247 | 2.045 | 1.879 | 2.879 | 2.498 | ---- | 2.475 | 2.625 | 1.978 | 1.574 | 2.482 | 2.062 | 2.802 |
| MM3T7 | 2.722 | 0.741 | 0.635 | 0.635 | 0.635 | 2.137 | ---- | 2.725 | 2.550 | 0.212 | 1.745 | 2.751 | 1.587 |
| ZacS8 | 2.732 | 0.287 | 0.431 | 2.909 | 2.160 | 2.915 | 2.481 | ---- | 1.747 | 1.193 | 1.341 | 0.436 | 1.183 |
| Coy2Si9 | 3.470 | 1.194 | 1.614 | 2.614 | 2.475 | 1.732 | 2.236 | 2.128 | ---- | 2.833 | 2.310 | 1.300 | 2.200 |
| Tav1C10 | 2.641 | 2.931 | 2.300 | 1.343 | 2.950 | 2.003 | 2.146 | 2.952 | 2.470 | --- | 2.565 | 2.647 | 2.891 |
| PotrS11 | 2.273 | 2.100 | 2.591 | 2.591 | 2.436 | 1.903 | 1.631 | 2.451 | 2.578 | 2.756 | ---- | 1.478 | 2.478 |
| Za2T12 | 1.777 | 2.887 | 2.990 | 0.888 | 2.443 | 2.762 | 3.546 | 2.330 | 3.542 | 2.665 | 1.665 | ---- | 2.994 |
| Ag2T13 | 0.872 | 2.378 | 2.865 | 2.865 | 2.702 | 2.084 | 0.996 | 0.872 | 2.084 | 0.996 | 0.872 | 2.084 | --- |
Results, from the Bayesian clustering analysis showed that the maximum posterior likelihood [LnP (D)] and the maximum ΔK value showed that K=4 is the effective number of genetic groups (Fig. 1, Fig. 2). Also, the DAPC analysis (Fig. 3) shown the same clustering and corroborates the grouping detected with STRUCTURE. Clusters detected by the DAPC in Fig. 2 and 3 show the distribution of ancestry proportions in each collection site. Cluster 1 (blue) is moderately widespread across the Oaxaca basin and includes the Guelaguichi (wild), Potrerillo (wild) and el Coyul (wild) populations. Cluster 2 (green) is mostly restricted to the Oaxaca mountains and includes populations of Taviche2 (cultivated), Taviche3 (cultivated), Taviche1 (cultivated), Agua de Sol2 (homegarden) and Agua de Sol1 (homegarden) which are consistently structured across the landscape. Cluster 3 (yellow) is restricted to the Oaxaca basin, and includes the populations of El Coyul (homegarden) and Zaachilac2 (homegarden). Cluster 4 (red) has a moderated geographic distribution through the Oaxaca basin and includes the populations of Zaachilac1 (wild), Moro Mazatlan3 (homegarden) and Morro Mazatlan2 (wild) 1. Accordingly with the great structure shown that the clusters are very different from each other, the presence of admixture in the populations was scarcely evident (Fig. 2).
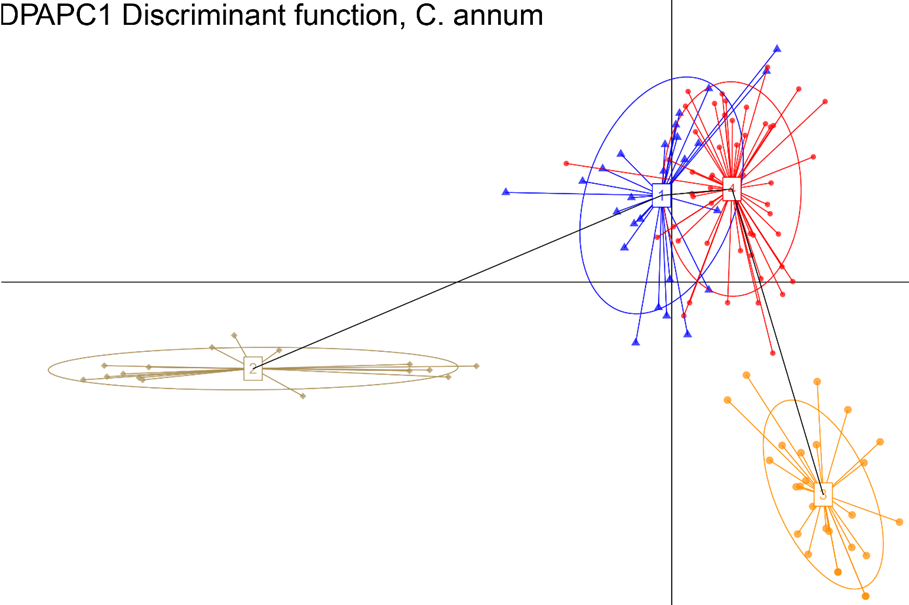
Inference of population structure as identified through DAPC. The corresponding BIC plot showing the optimal number of clusters (K = 4) and the DA eigenvalues for the displayed scatterplot.
Fig. 3 Discriminant Analysis of Principal Component, ordination based on 13 C. annum populations.
The results from genetic and geographic barriers analysis between 13 populations of C. annum, revealed two barriers (with over 50% bootstrap support) (Fig. 2). The most significant barrier, with 95% bootstrap support, sets apart the populations of Taviche2 (cultivated), Taciche3 (cultivated), Taviche1 (cultivated), Agua de Sol2 (homegarden) and Agua de Sol1 (homegarden), located in the northwest part of the basin from the rest of the C. annum populations. The second barrier, with 89% bootstrap support, is a complex break separating the southwestern part from the central part of the Oaxaca basin. This indicates that, in agreement with the high structure found, some populations have become isolated, such as the populations El Coyul (wild) from el Coyul (homegarden), Zaachila1 (homegarden) and Potrerillo (wild) (Fig. 2).
Testing for niche divergence and conservatism
C. annum genetic groups, had significantly non-equivalent ENMs (Fig. 4). The niche identity test indicated a high degree of climatic niche differences with values ranging from D statistic 0.774 to 0.837 genetic groups. The niche breadth measurements supported the hypothesis that broader niches exist for both C. annum: genetic groups 80%; 79%. These results indicate that the C. annum: genetic groups occupied different climate zones indicated by the niche between genetic groups (Fig. 4). The overlap index was a low: 0.225 between C. annum: genetic groups with low to moderate values of spatial range overlap (D = 0.261, I = 0.544) and indicated the amount of climate that share among the genetic groups in their distribution (Fig. 4).
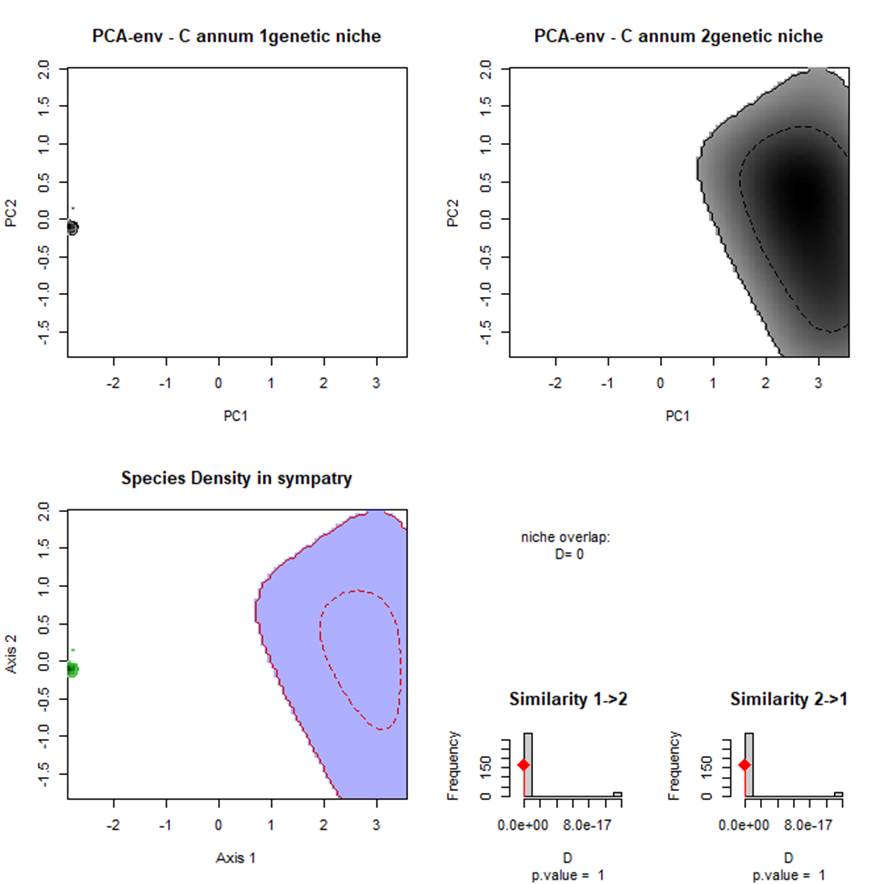
Fig. 4 Niche overlap densities plots using the PCA-Env scripts ecospat (Broennimann et al 2012) between environmental ranges of C. annum, for the different genetic groups (i.e. STRUCTURE) darker shading indicates higher density of occurrences of the species; solid contour lines, 100% of available environment; dashed contour lines, 50% of most common background environment. The available environment in the study areas are defined and by green and red lines when comparing the two species at the same time from the sympatric with populations with the same background area. The correlation circle shows the loadings of individual environmental variables to the two PCA axes andthe contribution of each variables to the construction of the PCA-Env. Sympatry.
Changes in population size
Results from the analyses to detect evidence of recent bottlenecks (excess heterozygosity) using the infinite allele (IAM), the two-phase (TPM) and the stepwise mutation (SMM) models see Table 4. No significant results were found with the IAM, TPM and SMM models as an indication of no bottlenecks in the wild, homegarden and domesticated populations of C. annum. Results from the test to estimate the effective population size (Ne) for C. annum populations, grouped according to domestication degree, shown that cultivated populations (Ne) = 96.2 individuals) had the highest value followed by the wild group populations (Ne) = 33.6 individuals) and homegarden populations (Ne) = 26.3 individuals). All Ne estimates had a high Jackknife support and a good confidence interval (CIs) (see Table 4).
Table 4 Bottleneck analysis for C. annum populations in the Oaxaca, Mexico using Wilcoxon rank test under infinite allele, stepwise mutation and two phase model. Parameters for TPM: variance = 10%, proportion of SMM= 90%, estimation based on 104 replications. P, probability. IAM, infinite allele model; TPM, two phase model; SMM, stepwise mutation model. ** Indicate significant deviation from equilibrium as value less than 0.05. The results obtained, for the estimation of the population effective size for the three genetic groups, values obtained with the program LDNe.
| Models | Wild | Homegarden | Cultivated |
|---|---|---|---|
| IAM | 0.01953 | 0.00781 | 0.71094 |
| TPM | 0.99219 | 0.40625 | 0.98828 |
| SMM | 1.00000 | 0.96094 | 0.99609 |
| LDNe | 33.6 | 26.3 | 96.2 |
Discussion
Domesticated species, have been exposed during their evolution to hundreds of years of human selection in multiple environments and cultural contexts. This leads to phenotypic diversity of fruits and seeds (Charlesworth et al., 1997). Native, homegarden and wild landraces could be affected by numerous factors such as, ecological characteristics, mountainous systems, anthropogenic disturbances and artificial selection (Larson et al., 2014; Renaut & Rieseberg, 2015).
A high genetic variation observed, was observed for C. annuum at the homegarden (H O = 0.833), whereas wild (H O = 0.728) and cultivated (H O = 0.620) showed lower values. Oaxaca state represents, a biodiversity hotspot in Mexico (Ferrusquía-Villafranca, 1993; García-Mendoza, 2004) and possess a great number of domesticated species and genetic diversity in regional chiles (Corona-Torres et al., 2000; Latournerie et al., 2001). A possible explanation of this diversity could be linked to the variability of soils, mountainous systems and the management of native and cultivated plants by farmers (Aguilar-Rincón et al., 2010; Pacheco-Olvera et al., 2012; López-Espinosa et al., 2018). Evaluation of genetic variability among the Capsicum genotypes result essential for selection. Assessment of such specific alleles and genotypes should be important, with respect to adaptability of crops, with respect to climate change and biotic and abiotic stresses (Pickersgill, 2007; Zizumbo-Villarreal & Colunga-García, 2010).
Genetic diversity reduction, has been observed in crops and is due to population bottlenecks and artificial selection (Charlesworth et al., 1997). In our C. annum populations, we detected low inbreeding and population size for wild (F IS = 0.244, Ne = 33.6), homegarden (F IS = 0.170, Ne= 26.3) and cultivated (F IS = 0. 121, Ne = 96.2) populations. We observed, for C. annum populations, a slightly reduction in inbreeding, effective population size and not significant signals of genetic bottlenecks (Larson et al., 2014; Renaut & Rieseberg, 2015). Domestication syndrome, signified the morphological traits shared by domestic, but not by wild ancestors. That could modified, reproductive traits such as, altered timing of flowering and altered color compounds. This syndrome, could affecting in isolated populations with self-fertilization, where little or no pollen flow is present (Contreras-Toledo et al., 2011).
We suggested for C. annuum the future tendency for landraces that will be exposed to the loss of diversity, affecting the ability to respond to climate changes (Meyer & Purugganan, 2013). Also, fire rates and habitat fragmentation has led chile plantations, wild, homegarden and cultivated to be isolated in patches (Ibiza et al., 2012). Depletion of genetic diversity, has been reported to increase susceptibility to infections, caused by fungi, (e.g. Phytophthora and Verticillium) (González et al., 2011). Crop wild relatives, contain genes to improve and resilience. Capsicum plants produced capsaicinoids that reduces the growth of fungal pathogens in wild chile, Capsicum chacoense (Rodríguez et al., 2007). Genetically modificated crops, are associated with various risks, such as, introduction of genes to susceptible crops, which leads to extinction of wild populations (Hanski & Mononen, 2011). Conservation programs, are necessary directed to select wild chile. Also gene flow, could contribute to rescuing genetic diversity of local varieties, that can counteract inbreeding and disease susceptibility (Corona-Torres et al., 2000).
Genetic structure and Bayesian admixture analysis
Our study found that, genetic structuring of C. annum among wild, homegarden and domesticated chiles, is associated with geographic features in the range of chile at the Oaxaca (Aguilar-Rincón et al., 2010; Pacheco-Olvera et al., 2012).
Oaxaca state represented, a biodiversity, cultural and ecosystems hotspot (Ferrusquía-Villafranca, 1993). Also harbored, a complex heterogeneity of soils, climatic that are delimited on a mountainous ranges coursing in a NNW-SSE direction and separated by valleys with tropical deciduous and xerophytic elements in the central portion (Ortiz-Pérez et al., 2004). These intricated, patterns resulted in a geographical barrier, contributing to isolation and genetic differentiation (García-Mendoza, 2004). We observed a great genetic diversity and differentiation that indicated, that domestication had an effect on the distribution of variation among domesticated chiles. Native chiles from Oaxaca, are an example of diversification of landraces used by humans such as, 'Tusta', 'Tabaquero', 'Solterito', 'Piquín', 'Nanchita', 'Costeño' and 'Chile de Agua' (Vera-Guzmán et al., 2011). Mountainous landscapes in Oaxaca, are constituted by heterogeneous topography and environmental ranges (Hanski & Mononen, 2011). Steep valleys and mountain demarcate the plant population habitats where gene flow is reduced thus, favoring differentiation (Latournerie et al., 2001). Genetic differentiation, could be incremented by drift associated with reduced population size (Pacheco-Olvera et al., 2012). Artificial selection, could be acting on plant populations determining morphological, physiological, reproductive, and genetic changes, leading to phenotypic and genotypic divergence between wild and managed populations (Kwon et al., 2005; Casas et al., 2007). As was observed, for local landraces of chile, which have been consumed the wild and domesticated chile across Oaxaca state (Zizumbo-Villarreal & Colunga-García, 2010).
The Cluster 1 (blue), Cluster 3 (yellow) and Cluster 4 (red) (e.g. Structure analysis) are located on the Pacific coast is associated with the Barrier 2 (Fig. 2). This clear structuring, have been revealed with populations with little gene flow. In the Sierra Madre del Sur, the valley ranges at elevations from 1000 m, but toward south elevations rise to 1800 m and host xerophytic vegetation and acts as a barrier to gene flow among tropical deciduous and temperate forest (Ortiz-Pérez et al., 2004). We suggest, that higher variation in habitat and climatic conditions combined with high elevations and geographic distances could explain genetic divergence (Larson et al., 2014). Populations in the valleys, are geographically isolated from highlands, thus gene flow is reduced and increasing differentiation. We indicated that in C. annum populations, a strong artificial selection could generate genetically diverse materials that has favored population adaptation to locations and environments (Hanski & Mononen, 2011; López-Espinosa et al., 2018). That could generate, diverse and divergent landraces adapted to different agroecological and cultural environments (Kwon et al., 2005; Zizumbo-Villarreal & Colunga-García, 2010). All those phenotypes producing fruit with the most desirable attributes according to local people are cultivated and represented the highest level of artificial selection (Alonso et al., 2008; Casas et al., 2007; Aguilar-Rincón et al., 2010).
Conclusions
High genetic diversity for C. annum at the Oaxaca state, that denoted a region with a great number of domesticated and richness in Mexico that could encouraging the domestication syndrome. Native chiles from Oaxaca, are an example of diversification of landraces used by human such as, 'Tusta', 'Tabaquero', 'Solterito', 'Piquín', 'Nanchita', 'Costeño' and 'Chile de Agua'. We indicate, that the degree of heterozygosity, allelic richness and private alleles could be used for improving. Evaluation of such genotypes should be important, with respect to adaptability of crops, with respect to climate change.
Low genetic inbreeding, for wild and homegarden and low effective population size for wild and cultivated. It is necessary, programs to rescuing chile varieties. Through In-situ, preserving economically important plants. Through Ex-situ, using Botanical Gardens. Also, gene flow, between communities could contributed to rescuing genetic diversity which can counteract inbreeding effects and disease susceptibility.
Genetic differentiation was moderate, for wild, homegarden and cultivated populations that underlined a clear genetic structuring. We suggest that for C. annum populations, a strong artificial selection, could generated genetically diverse materials that stimulated adaptation to locations and environments. Domestication or selection, had an effect on genetic and phenotypic variation. Oaxacan chile landraces, are in the stage of adaptation to different agroecological and cultural environments. As was observed, for local landraces of chile, which have been consumed, wild and domesticated at the Oaxaca state.











 nueva página del texto (beta)
nueva página del texto (beta)


