Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Sociedad Química de México
versión impresa ISSN 0583-7693
Rev. Soc. Quím. Méx vol.47 no.1 Ciudad de México ene./mar. 2003
Investigación
Development of Hydrophilic Ultrafiltration Membrane from Polysulfone-Polyvinylpyrrolidone
Heriberto Espinoza-Gómez1 and Shui Wai Lin2*
1 Facultad de Ciencias Químicas e Ingeniería, Universidad Autónoma de Baja California, Campus Tijuana, Calzada Tecnológico No. 14418, Mesa de Otay. C.P. 22390 Tijuana, Baja California, México.
2 Centro de Graduados e Investigación del Instituto Tecnológico de Tijuana, Apdo Postal 1166, Tijuana, Baja California México. Tel.+(664) 6233-772. E-mail: jhespinoza@hotmail.com
Recibido el 21 de enero del 2003;
acepado el 14 marzo del 2003.
Abstract
Ordinary polymeric membranes are hydrophobic in nature, thus hydrophobic polymeric membranes foul rapidly in water purification operations. A change in membrane surface properties can reduce fouling; this may be accomplished by increasing the hydrophilicity of the membrane surface, and by using a membrane with smaller pore size. The ultrafiltration membranes were prepared via phase inversion process in our laboratory, from polysulfone / polyvinylpyrrolidone (PS / PVP). The basic characteristics of these membranes like water permeability, water content and membrane selectivity were measured.
Key words: Ultrafiltration, Hydrophilic Membrane, Membrane Preparation and Structure.
Resumen
Las membranas poliméricas por naturaleza son hidrofóbicas, por tanto se ensucian rápidamente en operaciones de purificación de agua. Un cambio en las propiedades de las membranas puede reducir el ensuciamiento; esto se puede lograr al incrementar la hidrofilicidad de la membrana. Las membranas de ultrafiltración empleadas, se prepararon en nuestro laboratorio, por el método de inversión de fases, a partir de polisulfona/polivinilpirrolidona (PS / PVP). Se determinó la permeabilidad, contenido de agua, peso molecular límite, rugosidad superficial de las membranas y se estudio la sección transversal de las membranas
Palabras clave: Ultrafiltración, membranas hidrofílicas, preparación y estructura de membranas.
Introduction
Aromatic polysulfones are commonly used in the manufacture of synthetic membranes. They have outstanding oxidative, thermal hydrolytic, and dimensional stability. The inherent hydrophobic nature of polysulfone is often cited as disadvantage in using this polymer as a membrane material. Hydrophilicity can be imparted into polysulfone by the addition of hydrophilic functional polymer groups [1]. The overall characteristics of a membrane are dependent on its chemical structure, degree of asymmetry, crystalline lattice and morphology. Furthermore, the main characteristics in view of industrial development are both mechanical strength and resistance to chemicals, temperature, bacteria and to so-called "fouling" [2].
The demand for membrane separation systems is growing rapidly each year [3-5]. In spite of the success of membrane technology, membrane separation systems suffer from a serious problem: the membrane fouling [6]. Membrane fouling is caused mainly by (a) plugging the pore openings at the porous membrane surface by the suspended solid particles or large solutes in the feed and (b) the attachment of bacteria and subsequently colonization on the membrane surface [7]. For ultrafiltration membranes the biofouling can be minimized by periodical cleaning with sodium hypochlorite, by which the biofilm would be mostly washed away. The membrane system thus regains most of it is permeate flow rate. The membrane fouling due to plugging by solid particles or by large solutes is an irreversible process and ordinary cleaning methods do not recover the lost of permeate flow. The passage of water through these obstructed pore-openings is hindered and in order to maintain the permeate flow to a desired level, application of higher filtration pressure is needed.
Generally, proteins are adsorbed more strongly at hydrophobic surfaces than hydrophilic surfaces [7-9]. Initial biofilm formation is achieved by bacteria attachment through exopolymer synthesis at the membrane surface; this would be avoided if the membrane surface were hydrophilic in nature. In this study we had focused on how to improve membrane-fouling resistance by increasing the hydrophilicity of the neutral membrane.
The performance of an asymmetric polymeric membrane must be closely related to the morphology of the dense layer [10-12], where separation properties against solutes (the so-called cut-off) arise [13-15]. In this work we investigated neutral hydrophilic ultrafiltration membranes from Polysulfone / Polyvinylpyrrolidone (PS / PVP). In all of our membrane preparations we used 1-methyl-2-pyrrolidinone (NMP) as a solvent. Fig. 1 shows the chemical structures of the PS and PVP polymers. Our membranes were made by a phase inversion process.
Experimental
Materials
Dextran T-70 (70,000 Daltons) and Dextran T-500 (500,000 Daltons)-Pharmacia; Dextrans of Average Molecular Weights of 162,000 and 298, 000 Dalton-Sigma; 1-Methyl-2-pyrrolidinone (NMP)-Aldrich Chemical Company, Inc.; Polysulfone-Solutia; Polyvinylpyrrolidone-Solutia.
Instruments
Burleigh II Atomic Force Microscope; JSM5300 Scanning Electron Microscope; Technic's Hummer 5 sputter-coater.
Fabrication of Polymeric Membranes via a Phase Inversion Process
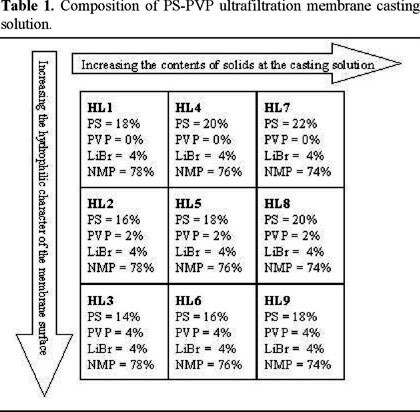
The compositions of membrane casting solution for the nine membranes of PS / PVP are shown in Table 1. Polymer components and LiCl (4-w/w %) were dissolved in 1-Methyl-2-pyrrolidone (NMP) in a clear bottle by tumbling under a heat lamp (~60 °C) until forming a uniform solution.
All the membranes used in this investigation were prepared via phase inversion process. The polymeric solution was smeared on a Texlon fabric laid flat on a glass plate; the casting knife gap was set at 152 µm (6 mils). Membrane casting speed was controlled by a D.C. motor and was set at 0.10 m / s (20 ft / min). Deionized water at 5 °C was used as coagulating solution.
Membrane Pure Water Determinations
The membrane sheets were thoroughly rinsed in distilled water to remove the remaining NMP solvent in the porous membrane and 3 × 8 cm2 (working area 22.12 cm2) coupons were used to determine the pure water flux and the "A-value" constant. Pure water permeate fluxes were measured 15 seconds after starting the cross-flow filtration operation under 275.6 Pa (40 psig) at 25 °C using distilled water as feed. The A-value is expressed in units of Kg / (Pa . m2 . s).
Molecular Weight Cut-Off (MWCO) Determination
Water, used for dextran solution preparation before each experiment, was purified using ion-exchange resin followed by distillation.
Dextrans of average molecular weights of 70, 162, 298 and 500 kDa were employed. The dextran solution was prepared by dissolving each individual dextran in 0.10 M NaCl solution buffered with phosphate at pH 7. The MWCO of each membrane was determined by the separation efficiency which is defined as R = (1 − Ci / Cp), where Ci is the concentration of the dextran present in the permeate and Cp is the concentration of the dextran in the feed. Test runs were carried out under an applied cross flow filtration pressure of 275.6 Pa at 25 °C. 1.0 cc of permeate was collected at 6, 12 and 16 min after the test run was started. The Refractive Index of permeate was determined 30 min after the permeate sample was collected. The content of the Dextran in the feeds and in the permeates was determined by a "Abbe-refractometer" (0 to 10 %) at 25 °C by measuring the Refractive Index of the permeates and the feeds against a blank and standard solution containing 0 % and 1.0 % dextran, respectively. The size of the membrane coupons used was 3 × 8 cm2 (actual membrane area = 22.12 cm2). The average MWCO of the test membrane is defined as the membrane sample having a 90 % or better rejection of the dextran in the feed [14-16].
Scanning Electron Microscopy (SEM)
The cross-sectional morphologies of the membranes were sputter-coated with gold using a Technic's Hummer 5 sputter-coated with a current of 15 mA for 3.5 min. The coated membranes were viewed with a JSM 5300 Scanning Electron Microscope, which was operated at an accelerating voltage of 10 keV.
In order to preserve the original dimensions of the pore and the porous structure of the membrane, the remaining water in the membrane was removed by a "Solvent Exchange" process which was carried out in the following manner. The wet membrane coupon was first soaked in pure isopropyl alcohol for 30 min; after that, the membrane coupon was sub-sequently soaked for 30 min in each isopropyl alcohol / hexane solution (75:25, 50:50 and 25:75). Finally the membrane was soaked in 100 % hexane for 30 min. The hexane within the membrane was dried under vacuum. Sample membranes to be examined by SEM were cut out and fractured in liquid nitrogen. The dried fractured membrane samples were sputtered with gold, and then the cross-sectional scanning electron micrograph of each membrane was recorded.
Atomic Force Microscopy (AFM) of polymer membranes
The surface morphologies of the wet membranes were characterized by contact mode with a Burleigh II AFM, equipped with a non-contact / contact head and a 100 m scanner, which was operated at a constant force mode (reference force 5nN). The wet membrane coupons were attached to a platinum sample holder that was mounted on the piezo scanner of the AFM. AFM images were acquired at a scan rate of 1.0-2.0 kHz and at an information density of 256 × 256 pixels (area 1mm2). The mean height is given by the average of the individual height determinations within the selected height profile.
Results
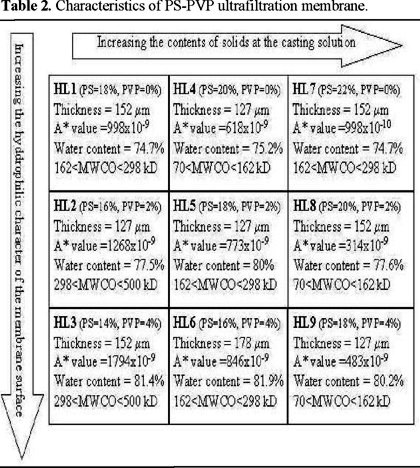
The experimental results indicate that the basic membrane characteristics depend on the compositions of PS/PVP membrane casting solutions. The trend of these changes are summarized in Table 2.
For a given horizontal row of membranes, as the wt-% of the total polymer in the membrane casting solution increases, the A-value and the molecular weight cut-off (MWCO) of the membranes decrease. For a given column of membranes, as the content of PVP increases, the A-value, the water content, and the MWCO of the membranes increases too. The membranes on the table do not show a consistent trend on the water content against the total polymer content.
In general, the pore volume in the final coagulated membrane is controlled largely by the concentration of polymer in the casting solution. Increasing the initial polymer concentration leads to a much higher polymer concentration at the interface. This implies that the volume fraction of polymer increases and, consequently, a lower porosity is obtained. Therefore, the pore size of the top layer can be controlled by changing the molecular weight of the polymer and polymer concentration.
As shown in Fig. 2, the scanning electron micrographs (SEM) reveal that at constant total polymer solid content and for high polymer solid content membranes (HL7, HL8 and HL9) the increase of polymer PVP wt-% in the membrane casting solution decreases the "finger print" of the cross-sectional structure of the membrane. Also for a given horizontal row of membranes, the size of the "void" and the "finger print" of the cross-sectional structure increases as the total polymer solid content increases.
We observed that the membrane surface roughness greatly depends on the composition of the membrane casting solution. As shown in the atomic force microscopy (AFM) images of this group of membranes (Fig. 3), all the HL membranes exhibited similar surface morphology consisting of a rough or mottled surface with well-defined holes or shallow depressions resembling pores or channels, respectively.
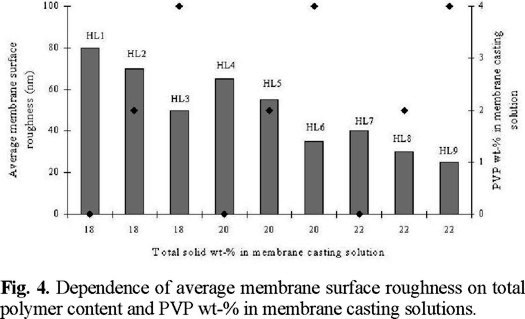
The plot of average membrane surface roughness and PVP wt-% in membrane casting solution versus total solid wt-% in membrane casting solution (Fig. 4) clearly indicates the effect of the total polymer content on the membrane surface roughness; for a given solid content, the pure PS membrane surface is rougher than the PS / PVP membrane surface; as the solid content in the polymer solution increases, the membrane surface becomes smoother.
Conclusions
The basic characteristics of PS / PVP membranes like water content, A-value, molecular weight cut-off, surface hydrophilicity, and surface roughness can be altered by addition of the desired polymer in the membrane casting solutions. For most ultrafiltration applications, the membrane is required to have a smaller molecular weight cut-off with narrow pore size distribution and highly hydrophilic surface. We believe that some of our membranes prepared in this investigation may find applications in the field of wastewater treatment processes like oil-water separation that requires membranes with highly hydrophilic surfaces.
Acknowledgements
The authors thank to Dr. Miguel Parra-Hake for valuable comments on the manuscript; to Mr. Israel Gradilla M. and Dr. Leonel Cota Araiza (from CCMC-UNAM Ensenada) for performing the SEM; and to M.C. Saul Zavala and Dr. Eugenio Méndez (from CICESE Ensenada) for performing the AFM. The authors are also grateful for the support provided by CONACyT (411074-5-28023-U) and also for the Scholarship of one of the authors (HEG).
References
1. Tremblay, A.Y.; Tam, C.M.; Guiver, M.D. Ind. Eng. Chem. Res. 1992, 31, 834. [ Links ]
2. Cappaneelli, G.; Vigo, F.; Munari, S. J. Membr. Sci. 1983, 15, 289. [ Links ]
3. Enoch, G.D.; Van den Broche, W.F.; Spiering, W. J. Membr. Sci. 1999, 87, 191. [ Links ]
4. Nguyen, Q.; Aotel, T.; Neel, P. J. Membr. Sci. 1980, 57, 71. [ Links ]
5. Krehbiel, D.K.; Scamehorn, J.F.; Ritter, R.; Christian, S.D.; Tucker, E.E. Separation Sci. & Tech. 1992, 27, 1775. [ Links ]
6. Applegate, L.E., Chem. Eng. 1984. [ Links ]
7. Han, W.; Gregor, H.P.; Pearce, E.M. J. Appl. Polym. Sci., 1999, 74,1271. [ Links ]
8. Knoell, T.; Safarik, K.J.; Cormack, T.; Riley, R.; Lin, S.W.; Ridgway, H. J. Membr. Sci. 1999, 157, 117. [ Links ]
9. Düputell, D.; Staude, E. J. Membr. Sci. 1993, 78, 45-00. [ Links ]
10. Kim, I.C.; Kim, J.H.; Lee, K.H.; Tal, T.M. J. Appl. Polym. Sci. 2000, 75, 1. [ Links ]
11. Kobayashi, T.; Fujii, N. J. Appl. Polym. Sci. 1992, 45, 1897. [ Links ]
12. Kobayashi, T.; Kumagai, K.; Nosaka, Y.; Miyama, H.; Fujii, N. J. Appl. Polym. Sci. 1991, 43, 1037. [ Links ]
13. Tremblay, A.Y.; Tam, C.M.; Guiver, M.D.; Dal-Cin, M.M. J. Chem. Eng. 1992, 69, 1348. [ Links ]
14. Kim, I.C.; Choi, J.G.; Tak, T.M. J. Appl. Polym. Sci. 1999, 74, 2046. [ Links ]
15. Sarbolouki, M.N. Sep Sci. & Tech., 1982, 17, 381. [ Links ]
16. Flegler, S.L.; Heckman, J.W.; Kloparens, K.L. Scanning and Transmission Electron Microscopy. An Introduction. USA, 1993. Chapter 7. [ Links ]




![Compuestos perazufrados de platino(II) con ditioéteres y tiolatos fluorados: Estructuras cristalinas de [Pt(SC6F5)2(p-C6H4FSCH2CH2-p-SC6H4F)], [Pt(p-SC6HF4)2(o-C6H4FSCH2CH2-o-SC6H4F)] Y [Pt(p-SC6HF4)2(p-C6H4FSCH2CH2-p-SC6H4F)]](/img/es/prev.gif)