INTRODUCTION
Rituximab is a monoclonal antibody against the CD20 protein present on the surface of B lymphocytes. It has demonstrated to increase the disease-free and overall survival (OS) of patients with non-Hodgkin lymphoma (NHL) CD20+1.
Previous reviews studying the infections associated with rituximab in patients with lymphoma, the addition of this drug to other chemotherapeutic agents did not result in an increased incidence of infections after more than 10 years of use; however, when used alone for maintenance for follicular lymphoma or in special populations (e.g., HIV-infected patients), rituximab comprised an additive risk factor for infections2. However, rituximab in combination with chemotherapy exerts cytotoxic effects on T cells that can lead to hypogammaglobulinemia and late-onset neutropenia, which have been related to higher risk of infection2. An increased risk has been reported for hepatitis B virus (HBV) and Hepatitis C virus reactivation1. Furthermore, a higher frequency of progressive multifocal leukoencephalopathy (PML) has been reported2. Other infections with a possibly increased occurrence associated are Pneumocystis jirovecii pneumonia; enterovirus encephalitis; Parvovirus B19; Cytomegalovirus; West Nile virus; parasitic infections, and mycobacterial infections2.
The incidence of infectious complications and the outcome of these events in rituximab-treated patients with NHL have not been acknowledged in middle-income countries, where there is a higher prevalence of tuberculosis (TB) and fungal infections (e.g., histoplasmosis). Thus, the spectrum of infections in patients in these countries will be expected to differ from that reported in literature on high-income countries. The aim of the study was to describe the incidence, spectra, infectious complications, and outcome in patients with NHL receiving rituximab-containing chemotherapy regimens. This is a retrospective study that included all patients from January 1, 2011, to December 31, 2012, who were diagnosed with NHL at the National Cancer Institute (INCan) who received at least one dose of rituximab.
MATERIALS AND METHODS
The INCan is a 135-bed referral teaching hospital located in Mexico City for adult patients with cancer, with an average of 170,000 medical visits, 7500 hospital discharges, and 3500 major surgical procedures per year.
This is a retrospective study that included all patients from January 1, 2011, to December 31, 2012, who were diagnosed with NHL and who received at least one dose of rituximab. The Governments program popular insurance, included patients with diffuse large B-cell non-Hodgkins lymphoma since 2011, and implemented standard chemotherapy plus rituximab at no cost, which is why almost all patients receive this combination. Patients with HIV infection were excluded from the study. The study was reviewed and approved by the Institutional Review Board (INCAN/CI/212/14).
Demographics, comorbidities, lymphoma characteristics (type, stage), chemotherapy, and number of doses of rituximab received were recorded. Patients were followed up from the first dose of rituximab received, up to 24 months after the last course of rituximab, or death.
An infectious episode was diagnosed when there was a microbiology-documented infection, radiographic or histological signs suggestive of an infectious process, or febrile neutropenia. Only infections Grades 3-5 of the common terminology criteria for adverse events were analyzed3. Absolute neutrophil and lymphocyte count, length of hospital stay, microbiological isolations, and outcome were registered for each infectious episode and at the end of the follow-up.
Main outcome was response to chemotherapy, classified as complete response (CR), partial response, progression, relapse, or death. Patients were considered lost to follow-up ≥ 6 months after their past medical visit. Positron emission tomography with 18-fluoro-D-glucose integrated with computed tomography (CT) is performed at lymphoma diagnosis and after finishing treatment (8 weeks after the last chemotherapy). At the middle of the number of cycles (third/sixth or fourth/eight), a CT-scan is performed. The number of chemotherapy cycles is considered in the base of the lymphoma stage: 6 for early and 8 for advanced.
Definitions
Clinically documented infection: fever accompanied by clinical findings.
Mucosal barrier injury bloodstream infection (MBI-BSI): patients who received chemotherapy with severe neutropenia (neutrophils < 500 cells/mm3 at least in two occasions or < 100 cells/mm3 at least on one occasion), or an allogeneic hematopoietic stem-cell transplantation, or Grade 3-4 gastrointestinal graft versus host disease, or diarrhea ≥ 1 L, or Grade 3-4 mucositis, and at least one blood specimen identified by culture with intestinal organisms4.
Pneumonia: radiological criteria on chest X-ray or a CT scan plus one or more of the following: fever (≥38°C) or hypothermia (<35°C); new cough with or without sputum production; pleurisy chest pain; dyspnea, and altered breathing sounds on auscultation.
Central-related bloodstream infection (CRBSI) was considered if time to positivity between blood cultures taken from the catheter was two or more hours apart from the blood culture taken from peripheral puncture, with signs of systemic infection (fever, chills, and/or hypotension), and no apparent source of infection except the catheter, and/or catheter-tip culture positive for the same organism (when the catheter was removed), and/or signs and symptoms of catheter entry-site infection5.
Neutropenic colitis required the presence of an acute abdomen with CT findings consistent with intestinal inflammation.
Urinary tract infection was defined as the coincidence of fever, typical symptoms, significant bacteriuria, and positive urine culture with an infectious pathogen.
Statistical analysis
Student t-test or MannWhitney U-test was used to compare continuous variables, and Chi-square or Fisher exact test was employed to compare categorical variables. A logistic regression analysis was performed for predicting risk for infection; variables with p < 0.5 on univariate analysis were included in multivariate analysis. Odds ratios with 95% confidence intervals were calculated. Overall Survival (OS) rates were estimated by the KaplanMeier method and the log-rank test. p ≤ 0.05 was considered statistically significant. Data were analyzed using STATA ver. 14 statistical software (Stata Corp., College Station, TX, USA).
RESULTS
During the study period, 265 patients were included: 108 (40.8%) men; mean age was 60 ± 15 years; 46 (17%) had diabetes mellitus (DM). An aggressive type of lymphoma (diffuse large B-cell n = 205, mantle-cell n = 4, Burkitt n = 1, or marginal-zone n =1) was documented in 211 patients (79.6%); 180 (67.9%) were in Stage 3 or 4 (Ann Arbor staging system), and 261 (98.5%) received standard first-line treatment as follows: rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). Other clinical characteristics are presented in Table 1.
Table 1 Demographic and clinical characteristics in patients with non-Hodgkin lymphoma who received rituximab in comparison with patients who developed an infection versus those who did not
| Characteristics n (%) | Non-infection n=180 (67.9) | Infection n=85 (32.1) | Total n=265 | p |
|---|---|---|---|---|
| Male patients | 71 (39) | 37 (44) | 108 (40.8) | 0.528 |
| Age* (years) | 60.8±15 | 60.3±15 | 60.5±15 | 0.961 |
| Above 60 years of age | 87 (48) | 45 (53) | 132 (50) | 0.484 |
| Diabetes mellitus | 31 (17) | 15 (17) | 46 (17) | 0.932 |
| Other cancers& | 14 (8) | 4 (5) | 18 (7) | 0.354 |
| Follow-up* (months) | 28.6±11.9 | 18.9±13.3 | 25.5±13.1 | <0.001 |
| Lymphoma type | 0.158 | |||
| Follicular | 41 (22.8) | 13 (15.3) | 54 (20.3) | |
| Diffuse large B-cell | 134 (74.4) | 71 (83.5) | 205 (77.4) | |
| Mantle cell | 3 (1.6) | 1 (1.2) | 4 (1.5) | |
| Burkitt | 1 (0.6) | 0 | 1 (0.4) | |
| Marginal zone | 1 (0.6) | 0 | 1 (0.4) | |
| Stage§ | 0.04 | |||
| I-II | 62 (34.4) | 19 (22.3) | 81 (30.6) | |
| III-IV | 118 (65.6) | 66 (77.7) | 184 (69.4) | |
| First-line CT | 0.760 | |||
| CHOP/COP | 177 (98) | 84 (99) | 261 (99) | |
| Other | 3 (2) | 1 (1) | 4 (1) | |
| Failure to first CT scheme | 25 (13.9) | 31 (36.5) | 56 (21.1) | <0.001 |
*Median±Standard deviation,
&There were 18 patients with another neoplasia: 5 synchronic and 13 non-synchronic (four thyroid carcinomas, five skin neoplasia [three basocellular and two epidermoid], two breast cancers, two cervical cancer, one vulvar, one hepatocellular carcinoma, one myeloid leukemia, one meningioma, and one teratoma).
§Stage was described in 258 patients (in seven, the staging was not performed), CT: computed tomography, CHOP: cyclophosphamide, doxorubicin, vincristine, and prednisone; COP: cyclophosphamide, vincristine, and prednisone.
Eighty-five patients (32%) developed a Grade 3-5 infection; incidence rate was 41.98 infections per 100,000 person-days. There were 177 events in the 85 patients, and 42 (49%) of these had more than one event, (median, 2 events/person; interquartile range [IQR], 1-3 events). There were significantly more patients with an infection that did not achieve CR with the first chemotherapy scheme (n = 31, 36.5%) versus those without infection (n = 25, 13.9%; p < 0.001). There were no other significant differences in baseline characteristics between patients who developed an infection versus those who did not develop one (Table 1).
Median time to infection since day 1 of therapy was 133 days (IQR, 53-270 days). Most common infections comprised febrile neutropenia (n = 38; 21.5%); MBI-related infections (n = 28; 15.8%), and pneumonia (n = 38; 21.5%). Other infections and outcomes are depicted in Table 2.
Table 2 Frequency of infectious events and outcome in patients with non-Hodgkin lymphoma who received rituximab
| Infection n(%) | Total (n=177) | Death related with infections (n=35) |
|---|---|---|
| Pneumonia | 38 (21.5) | 14 (40) |
| Febrile neutropenia | 38 (21.5) | 0 |
| Mucosal barrier injury BSI* | 28 (15.8) | 7 (20) |
| Urinary tract infection | 19 (10.7) | 1 (2.8) |
| Skin and soft tissue infection | 17 (9.6) | 5 (14.2) |
| Abdominal sepsis | 12 (6.8) | 3 (8.5) |
| CRBSI& | 16 (9) | 3 (8.5) |
| Meningitis | 2 (1.1) | 0 |
| Other | 7 (4) | 2 (5.7) |
*BSI: bloodstream infection,
&CRBSI: catheter-related bloodstream infection.
In 88 of the events (49%), there was a microbiologic diagnosis; the most frequent were bacteria (n = 73; 41.2%) (Fig. 1). 10 patients (5.6%) had a virus (five varicella-zoster, one coronavirus, one metapneumovirus, one influenza virus, and one papillomavirus); six (3.39%) had fungi (four Candida spp. and two Aspergillus spp.); four (1.5%) had Mycobacterium tuberculosis complex (incidence rate 721/100,000 person-year), and one patient had a parasite (Strongyloides stercoralis). In 86 cases (48.6%), there was not a microorganism identified: 38 cases (21.5%) with fever and neutropenia, 16 (9%) with MBI, 14 (7.9%) with pneumonia, 5 (2.8%) with skin and soft tissue infection, 6 (3.4%) with abdominal sepsis, 2 (1.1%) with gastroenteritis, 1 (0.6%) with encephalitis, and 4 (2.3%) with other infections.
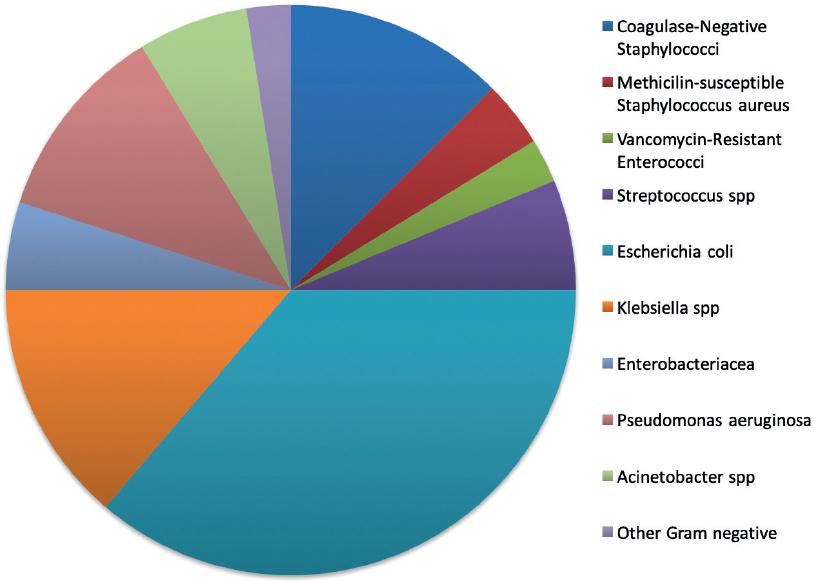
Figure 1 Microbial characteristic of bacteria isolated from patients with non-Hodgkin lymphoma who received rituximab (n = 70).
None of the patients were positive for HBV surface antigen (HBsAg) at baseline screening, there were six patients with positive anti-core HBV (anti-HBc) who did not receive pre-emptive antiviral treatment, and none of these patients had HBV reactivation during follow-up.
We did not find any case of histoplasmosis, despite the fact that Mexico has a high prevalence of this infection. Probably there is an underestimation, due to the fact that blood marrow cultures are not performed as a routine unless there is suspicion of an infection that can be documented by this study.
Median follow since first rituximab dose was 18.9 ± 13.3 months. There were not severe adverse events related to rituximab. During follow-up, 71 patients died (27%); in 35 cases (49.3%), the cause of death was infection: bacteria were isolated in 17 cases (48.6%), fungi in two (5.7%), virus in two (5.7%), and in 14 cases (40%), there was not a microorganism identified. The most frequent death-related infections were pneumonia (n = 14; 40%), MBI-BSI (n =7; 20%), and skin/soft tissue infections (n = 5; 14%) (Table 2). Mortality between patients who developed an infection event and those who did not is shown in figure 2. Of all the patients who died, 65 (91.5%) were in progression or relapse, compared with 21 (10.8%) of those who survived (p < 0.0001). There were no differences when compared those patients with progression or relapse who died related with an infection (n = 33, 94.3%) compared with those who died due to another cause (n = 32, 88.9%; p = 0.673).
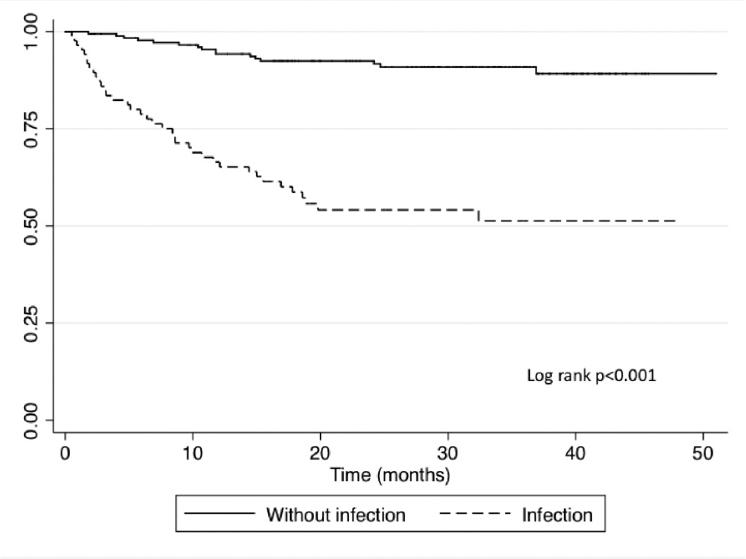
Figure 2 KaplanMeier overall survival curve in patients with non-Hodgkin lymphoma who received rituximab compared with those who developed an infection versus those who did not.
Multivariate analysis for mortality revealed that age > 60 years, failure to achieve CR, and development of an infectious complication increased the risk of death (Table 3).
Table 3 Multivariate analysis of factors associated with mortality patients with non-Hodgkin lymphoma who received rituximab
| Risk of death | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Alive n=194 n (%) | Death n=54 n (%) | Univariate | Multivariate | ||
| OR (95% CI) | p | OR (95% CI) | p | |||
| Age (year) | 0.001 | |||||
| <60 | 110 (57) | 16 (30) | 3.1 (1.6-5.9) | <0.001 | 1.00 3.7 (1.7-8.2) | |
| >60 | 84 (43) | 38 (70) | ||||
| Achieve complete response | 0.001 | |||||
| Yes | 173 (89) | 4 (7) | 102.9 (33.7-313.9) | <0.001 | 1.00 3.9 (1.8-8.8) | |
| No | 21 (11) | 50 (93) | ||||
| Infectious complication | <0.001 | |||||
| No | 153 (79) | 16 (30) | 8.8 (4.5-18.6) | <0.001 | 1.00 7.9 (3.8-17) | |
| Yes | 41 (21) | 38 (70) | ||||
CI: confidence interval, OR: odds ratios.
DISCUSSION
In this study, we report 177 infections in 85 patients NHL who received rituximab and were followed for a mean of 18.9 months. Only in 50% of the cases, there was a microbiology etiology, being bacteria the most frequent cause (39.6%) followed by virus (5.6%) and TB (2.3%).
In a recent report that included 101 patients treated with rituximab (with autoimmune and hematological diseases), 73.3% presented infectious events6. Other studies which have included only patients with hematological diseases, the incidence of serious infections was reported between 50 and 58%: 11% were opportunistic pathogens and 13% were fatal episodes6,7. These data are similar to the results we found: rate of serious infection was 66.8%, incidence rate for opportunistic pathogens 6.8%, and for the fatality rate associated with infection 13.2%. The lower percentage of opportunistic infections that we found could be explained as due to the lack of molecular tests to identify non-cultivable or difficult-to-grow pathogens at our center.
Considering the etiology of infections reported in patients with lymphoid malignancies treated with rituximab have been described: 31% for bacterial infections, 10% for viral infections, and 1% for fungal infections8. In a systematic review of 944 patients, of whom 535 received rituximab, overall number of opportunistic infections diagnosed was 299 (31.7%), 109 (36.5%), of unknown or unspecified origin; 100 (33.4%), viral reactivations; 44 (14.7%), bacterial; 26 (8.7%), fungal; 11 (3.7%), protozoal, and nine (3%), viral infections9. In the present series, nearly one-half of the episodes were of unknown origin, in 39.6% a bacterium was identified, in 5.1% a virus, in 2.3% a fungus, in 2.3% a mycobacterium, and in 0.6%, a parasite was identified, without viral reactivations.
Since 2002, an increase of cases of PML has been described as being related to rituximab use10. In this report, we did not find any case of PML.
The screening protocol carried out in our hospital for more than 10 years and all patients diagnosed with lymphoma, is to perform serological tests for HIV, HBV, and HCV, since patient admission. Before carrying out this study, we did not have an established protocol for latent TB infection (LTBI). At present, tuberculin test purified protein derivative (PPD) is performed in all NHL patients who will receive rituximab; and in those with > 5 mm, a CT thorax is done to evaluate if they are candidates to receive isoniazid 300 mg QD for 9 months. Although the majority of individuals infected with M. tuberculosis do not develop active disease, they harbor the dormant mycobacterium as a LTBI. The mycobacteria are confined by granuloma through recruitment of CD4 and CD8 T cells, B cells, and macrophages to the infected site. Rituximab can cause active TB by disrupting the granuloma11. Some studies have underestimated the TB prevalence rate associated with different biological therapies11. The prevalence found in this group of patients was 1.5% (721/100,000 incidence rate), considerably higher than TB incidence reported by the World Health Organization for Mexico (21/100,000 population-year)12. An important point to consider is the relationship that exists between TB and DM13, and the high prevalence of DM that exists in Mexican population. However, none of the four patients who developed mycobacterial infection had a diagnosis of DM. The high incidence rate reported in our study, in the first 2 years of receiving rituximab, warrants the recommendation to investigate LTBI and to prescribe prophylaxis in patients expected to be treated with rituximab.
There were not patients with HBV reactivation. Although we did not perform HBV-DNA in all patients, none had HBsAg positive. In those patients who presented transaminases elevation, HBV serology was repeated, and no patient had HBsAg seroconversion.
We did not include patients with HIV in this study; these individuals comprise a special group with a higher risk for infections (around 12%), particularly opportunistic pathogens in patients with CD4 of fewer than 50 cells/mm39,14.
There were more patients with an infection that did not achieve complete tumor response with the first chemotherapy scheme when compared with those without infections (p < 0.0001). This could be related to a delay to receive timely cycles or a decrease in chemotherapy dosing.
Eighteen patients presented with some neoplasm: 13 asynchronous and 5 synchronic. During the follow-up, no patient developed a new neoplasm, which was probably related to the short median follow-up time (19 months).
Likewise, infectious processes, along with age above 60 years, and failure to achieve CR were risk factors associated with mortality. A study that included 325 patients with diffuse large B-cell lymphoma who received rituximab and who was followed for 10 years, the incidence of infection was 63.4%. Infection was an independent predictor of death, in addition to age, the Charlson Comorbidity Index, Eastern Cooperative Oncology Group performance status, and neutropenia15. In the past years, several infectious agents have been reported as involved in the malignant transformation of B or T lymphocytes, and therefore associated with the pathogenesis of lymphoproliferative disorders16. Furthermore, the antigenic infectious stimulation was shown to be fundamental in the cell perturbation of the microenvironment in sustaining the neoplastic cell growth, which can condition failure in response to oncology treatment16. Although this hypothesis is interesting, this study does not intend to establish that infections stimulate or condition a favorable environment for tumor growth.
Study weaknesses include that data derived from a single oncology center; therefore, these data may not apply in the same way to other populations, in that local epidemiology and local policies may vary. PPD was not performed in all patients, so no timely diagnosis of LTBI was made, and therefore prophylactic treatment was not prescribed before the start of rituximab. All the patients had a baseline serological study for hepatitis; however, this was not repeated continuously either semi-annually or annually to assess if seroconversion occurred; it was only performed in those with elevated transaminases to rule out infection or reactivation of viral hepatitis. Rituximab doses can be different between different lymphoma subtypes; however, the cumulative doses have a long-term effect. Lack of molecular testing for some pathogens is one of the main problems that the majority of government hospitals face in middle and low income countries.
The studys strengths are the inclusion solely of patients with lymphoma; thus, we had a captive population that allowed us to follow them. To emphasize the importance of conducting screening tests for TB in all patients who will receive rituximab. Moreover, a continuous follow-up even when they have finished the chemotherapy scheme for at least 2 years.
The most important finding was the development of infectious complications in patients with NHL receiving first-line chemotherapy with R-CHOP which increased the risk of mortality.











 nueva página del texto (beta)
nueva página del texto (beta)


