Introduction
The Neotropical otter (Lontra longicaudis annectens) has a wide distribution range in México; it can be found in the Pacific slope and coast including the mainland coasts of the central and southern Gulf of California, in the Gulf of México slope and coast, and in the Caribbean slope; the species inhabits in many and diverse habitat types along creeks, rivers, lakes, lagoons, wetlands, and reservoirs (Gallo-Reynoso 1997; Briones-Salas et al. 2008; Hernández-Romero 2016; Gallo-Reynoso and Meiners 2018). The species’ altitudinal range spans from sea level to 2,500 masl and inhabits river basins across zones covered by different vegetation types including coastal marshes, mangrove forest, thorny scrubland, tropical deciduous forest, tropical evergreen forest, mountain cloud forest, and oak-pine forest (Gallo-Reynoso and Meiners 2018; Hernández-Romero et al. 2018).
The Neotropical otter is listed as endangered species in México (NOM-059-SEMARNAT-2010; SEMARNAT 2019) and is included in the Agreement for Priority Species and Population Conservation in México (SEMARNAT 2014). It is listed under subsection I of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES 2017) and in the red list of the International Union for Conservation of Nature (IUCN 2019).
In Yucatán, due to the karstic nature of the soil, surface water bodies are relatively shallow and streams flow underground with water outcrops and wells locally called cenotes (sinkholes). Cenotes may be of various types: the most typical are cavern-type cenotes with stalactites and stalagmites, but some are open cenotes, similar to small lagoons (Cervantes-Martínez 2007). This particular freshwater system in Yucatán favors suitable habitats for the Neotropical otter and may foster the habitat connectivity required for the species to establish viable populations, since otters have been recorded in Campeche, Quintana Roo, and Yucatán (Gallo-Reynoso 1997; Sánchez and Gallo-Reynoso 2007; Calmé and Sanvicente 2009; Santiago-Plata et al. 2013; Sosa-Escalante et al. 2013; Gallo-Reynoso and Meiners 2018; Vazquez-Maldonado et al. 2021).
It is worth to mention that time gaps between records in the same localities in the State of Yucatán may indicate preferential habitat types (Sosa-Escalante and Martínez-Meyer 2014). For example, the first recorded occurrence of Neotropical otter in Yucatán was reported by Leopold (1965) at Ría Celestún, 64 km W Mérida. The presence of specimens at Ría Celestún Biosphere Reserve was confirmed in 2000 (Sánchez and Gallo-Reynoso 2007). Thus, the species was recorded on two different occasions separated by circa 35 years (1965, 2000) in the same natural protected area. Although the Neotropical otter is mentioned as present in the Management Program of Ría Celestún Biosphere Reserve, there are no reports available of the species conservation status (CONANP 2000). From the above, it follows the need to investigate if the previous records of Neotropical otter at Ría Celestún, and the record of 2000 at San Crisanto in Yucatán (Leopold 1965; Sánchez and Gallo-Reynoso 2007) may correspond to marginal (i. e., isolated) populations, and/or if the species might be facing serious conservation issues in Yucatán. Thereby, it is important to identify the potential distributional range and potential suitable habitat for the Neotropical otter in Yucatán, to confirm its current distribution. The objectives of this study were to generate a model of the potential distribution of the Neotropical otter in the State of Yucatán and verify the otter’s presence in sites predicted by the model.
Materials and Methods
Study Area. The Yucatán peninsula is located in southeastern México and separates the Gulf of México to the north and west from the Caribbean Sea and the northern part of Central America to the east; Yucatán is on the northern part of the peninsula, between Campeche and Quintana Roo. Climate is subhumid with summer rains, mean annual precipitation of 1,600 mm and mean annual temperature of 26°C; the dry season spans from March to May and the rainy season from June to October (García 2004; Estrada and Cobos 2014). Dominant winds from the north prevail from November to February, with a tropical cyclone season (tropical storms and hurricanes) from August to November (Herrera-Silveira 2006; INEGI 2008). Extensive areas in the Yucatán peninsula are covered by deciduous and evergreen tropical forests; nevertheless, Yucatán also have various vegetation communities that include coastal dune vegetation, mangrove forest, floodplain forest, savannah, wetland vegetation known locally as petén, popal, tular, and secondary vegetation (Flores-Guido et al. 2010; Pérez-Sarabia 2017; Zavala-Cruz et al. 2016).
A calcareous platform made up of a series of plains and plateaus of karstic origin with underground drainage constitute the State. The northern part has low elevations, less than 250 m, except for the Sierra de Ticul with an elevation of 350 masl (Herrera-Silveira 2006; Pérez-Sarabia 2017). In the southern part of the Yucatán and the area bordering with Campeche and Quintana Roo, the relief consists of plateaus and karstic valleys of recent formation (Herrera-Silveira 2006; Pérez-Sarabia 2017). The underground system connects areas of water recharge within deciduous and medium tropical forests with coastal areas with mangrove forests where cenotes are also located (Estrada-Medina et al. 2014). The areas where karstic rock has dissolved by water filtration through geologic time gave place to large cavern-type cenotes (Estrada-Medina et al. 2014). Some cenotes are deep, closed caverns; other cavern-type cenotes are partially open where portions of the cavern roof have collapsed; and cenotes with roofs that collapsed completely in the past are now fully open and exposing the underground water (Estrada-Medina et al. 2014).
Rainwater permeates underground due to the carbonate rocks of the peninsula platform, as well as through abundant fractures in the karstic rock which hamper the formation of surface currents like creeks or rivers. As such, a few rivers are formed in the borders between Yucatán, Campeche, and Quintana Roo, increasing in quantity in southern Campeche towards Tabasco, the same is presented in southern Quintana Roo towards Belize, and connecting with many coastal lagoons and deltaic systems (Zavala-Cruz et al. 2016; Pérez-Sarabia 2017).
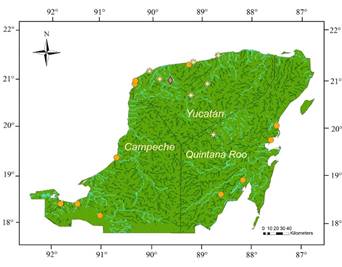
Figure 1 Potential distribution of the Neotropical otter habitat in Yucatán (Dark green), with historical records of this species (orange circles); from Sánchez and Gallo-Reynoso (2007) and Gallo-Reynoso and Meiners (2018). Favorable environmental sites in blue. Surveyed sites are indicated (yellow stars) for seven localities: San Crisanto, Cenote Tunkás, Yalahau Lagoons State Park (Parque Estatal Lagunas de Yalahau), Chichankanab Lagoon, Sisal, San Antonio Chel, and El Islote (Dzilam de Bravo). Binary map. Coordinate System WGS84. Environmental layers from Earth Env. Domisch et al. 2015.
Some freshwater springs arise inside mangrove forests and petenes, as well as surface water reservoirs called “aguadas”, which are topographic depressions that allow rain to be accumulated due to a fine layer of compacted clay sediment that prevents the filtration of rain to underground waters. Another type of spring water is called “sartenejas”, which are reservoirs of underground water, some of which have been used by the local Maya inhabitants to excavate and build cisterns with wide fringes to delimit water sources and channels to conduct rainwater (Herrera-Silveira 2006).
Potential distribution map. We gathered georeferenced observations from international data sources GBIF (Global Biodiversity Information Facility 2016) and VertNet (Constable et al. 2010), from national collections on biodiversity (e. g., the online catalogue of the Coleccion Nacional de Mamíferos (CNMA) of the Instituto de Biología, Universidad Nacional Autónoma de México and from scientific literature such as articles and reports (Martínez-Meyer and Sánchez-Cordero 2006; Sánchez and Gallo-Reynoso 2007; Guzmán-Soriano et al. 2013).
The potential distribution of the Neotropical otter was modeled considering the georeferenced otter records and environmental variables that favor its presence in Yucatán. Spatial autocorrelation was eliminated from the record database with the geographic information system ArcMap 10.2 (produced by ESRI 2014) and the tool “Spatially Rarefy Occurrence Data for SDMToolbox” (Brown 2014) to prevent over-adjustment in areas subjected to more intensive sampling (Veloz 2009; Boria et al. 2014; ESRI 2014b). The refined database was split into two subsets: 1) correspond to species records within its geographic range, excluding Yucatán (hereafter ‘calibration base’) and 2) that considered the records only for the Yucatán peninsula Biotic Province or YPBP (hereafter ‘evaluation base’). The two subsets were defined to have independent records of the species to evaluate the estimated environmental suitability and the potential distribution area. As predictors of the potential distribution modeling, several environmental variables from EarthEnv (Domisch et al. 2015) were used, which contain suitable information on freshwater organisms at ~1 km² resolution (Table in Appendix 1). All variables were delimited accordingly with the area of the two scenarios mentioned above. A MaxEnt algorithm (Maximum Entropy; Phillips et al. 2006) were used to generate the model because it requires presence data only and allows the use of categorical variables and flexible parameterization (Elith et al. 2011).
The calibration area of the model was delimited from the shapefile of Terrestrial Ecoregions of the World (Olson et al. 2001); from it, a polygon was drafted representing the hypothesis of historic accessibility for Neotropical otter populations, constituted by the ecoregions that contained records of L. longicaudis annectens within the Neotropical region (‘M’ area in the BAM diagram; Soberón and Peterson 2005). M was partitioned in two polygons according to the data subsets previously split into two stages: ‘stage one’, which included the complete distribution range of the species, excluding Yucatán, which corresponded to the ‘calibration base’; and ‘stage two’, which included the Yucatán peninsula, corresponding to the ‘evaluation base’.
The model was calibrated with the information from stage one using the observations from the calibration base as inputs. Then, the model was transferred to stage two (Yucatán) to represent the environmental suitability for Neotropical otter in Yucatán based on environmental characteristics of its distribution range for the whole country. The effectiveness of the model was corroborated by using the projection of the evaluation base in the resulting model for stage two; and to validate the models, an analysis of the partial ROC curve was performed, which was configured to perform 1000 “bootstrap” tests using the iPartialROC function (Barve 2008) from the ENMGadets package in R (R Development Core 2017). To estimate the potential distribution of the species, we reclassified the data from the MaxEnt model and generated binary probability models (absence/presence), using ArcMap 10.2.
Otter’s presence verification in the field. In October of years 2017, 2018, 2019 and 2021, we visited a total of seven sites which included coastal ecosystems of marshes and mangrove forests, cenotes, and epicontinental lagoons (Table 1). The sites were selected accordingly to the presence of suitable habitat predicted by the potential distribution map previously generated (Figure 1).
Otter vocalizations sessions were held in locations where the presence of otters was predicted by the model. Previously, we recorded otter vocalizations consisting of barks and high-pitched barks of an individual otter from the Miguel Álvarez del Toro Zoo, in Tuxtla Gutierrez, México. These vocalizations were played every 10 minutes, for one minute, during the surveys, until there was a response or after 30 minutes with no response.
In every visit, we recorded geographic data with a GPS (Garmin GPSmap 78s) to obtain locations, distances, routes, altitude, and survey effort. The perimeter of cenotes, coastal lagoons, continental lagoons, marshes, and wetlands was surveyed on foot and/or navigating on a canoe or a kayak, searching for direct sightings and indirect signs of Neotropical otter’s presence. At the seven sites surveyed, habitat characteristics were described, and the habitat requirements of Neotropical otters were verified including freshwater presence, a stable water level, water transparency, subaquatic vegetation, presence of suitable prey types inside and outside of the water body, tree coverage, and suitable areas to establish breeding and resting dens like rocky areas and holes excavated in banks of cenotes or lagoons, or among dense vegetation.
The information on tracks, footprints, latrines, and dens was recorded in a database. We collected biological samples and specimens (spraints, anal jellies, food residues, and plant specimens), and recorded photographic and video material that showed the characteristics of the studied areas. With a GoPro Hero2 video camera, we recorded underwater video to understand the underwater habitat characteristics such as presence of underwater vegetation, species of fish present and features of each site such as the depth of water, as well as otter vocalizations. Collected materials were deposited at the Laboratorio de Biología de la Conservación at the Parque Científico y Tecnológico de Yucatán. Also, in the surveyed areas, we searched for connection between water bodies (i. e., several lagoons that were separated by 500 to 1,000 m) that would facilitate the movement of Neotropical otters.
Results
Otter’s potential distribution. The potential distribution of the Neotropical otter in Yucatán covered an estimated area of approximately 3,487 km2, equivalent to 8 % of the area of Yucatán and 2.6 % of the Yucatán peninsula. The potential distribution modeling allowed us to identify suitable habitat for the Neotropical otter in inland areas of the Yucatán peninsula; although most known records were located near the coast in mangrove forest and wetland vegetation known as petén, popal, and tular. Seven sites predicted with potential habitat for otter in Yucatán were visited to confirm the presence of otters.
Otter’s presence verification in the field. San Crisanto (site 1). With a total survey effort of 5 km, the paths between the artisanal salt evaporation plots were checked on foot, the mangrove area was not visited because it was flooded by the high tide. The vegetation consisted of mangroves (Rizophora mangle, Laguncularia racemosa), and coastal dune vegetation (shrubs and grasses) in the salt evaporation plots, particularly a pioneer vegetation zone (Soriana maritima, Sesuvium portulacastrum, Sporobolus virginicus, Distichlis spicata).
Feeding sites were found with presence of bones, feathers, and wings mostly of Blue-winged Teal (Spatula discors); one site presented remains of an American flamingo juvenile (Phoenicopterus ruber). We found a series of footprints and tracks of Neotropical otter in one of the clay shores of the marsh and a spraint on the vegetation (Figure 2).
Table 1 Characteristics of the seven sites visited to verify the Neotropical otter’s (Lontra longicaudis annectens) presence in areas with predicted suitable environmental conditions in the State of Yucatán, southeastern México.
| Site name, geographic coordinates, and location | Site description |
|---|---|
| 1. San Crisanto. 21° 20.914 N, -89° 10.281 W. 51 km E of the city of Progreso, north Yucatán. | Coastal locality with marshes, wetlands, mangrove forests, cenotes, estuaries, and coastal areas, as well as evaporation parcels for obtaining artisanal salt within a mangrove preservation zone. San Crisanto Mangroves Management Unit for Wildlife Preservation (Unidad de Manejo para la Conservación de Vida Silvestre [UMA] Manglares de San Crisanto [SEMARNAT-UMA-EX0196-YUC-11; 21.34735 N, -89.18113 W]). |
| 2. Cenote Tunkás. 20° 54.558 N, -88° 52.013 W. 30 km W of Cenotillo. | Open cenote with an approximate diameter of 75 m. This water body is located within a complex of 20 cenotes, lagoons, and aguadas located in the northeastern portion of the Yucatán State where large-scale tourism is not yet developed. |
| 3. Yalahau Lagoon. 20° 39.435 N, -89° 13.034 W. Yalahau Lagoons State Park, 60 km SE of the city of Mérida. | Yalahau Lagoon in the main lagoon of Yalahau Lagoons State Park, a Yucatán State Natural Protected Area encompassing three epicontinental lagoons, cenotes and aguadas located in the central part of the State of Yucatán (DOF 2004). |
| 4. Chichankanab Lagoon. 19° 52.113 N, -88° 45.886 W. 150 km SW of the city of Mérida. | Chichankanab Lagoon is located at the central-west portion of the State of Quintana Roo, limiting with the State of Yucatán, and is approximately 30 km long stretching from La Presumida to Kantemó. Chichankanab is the largest epicontinental lagoon system of the Yucatán peninsula. |
| 5. Sisal. 21° 09.554 N, -90° 02.930 W. 53 km NW of the city of Mérida. | Sisal is near Punta Piedra in a tide-flooded area with gravel bottom, several kilometers away from the “Paraíso Sisal” residential complex. A portion of the wetland is part of the “El Palmar” State Ecological Reserve. |
| 6. San Antonio Chel. 20° 59.903 N, -89° 50.659 W. 7.6 km east of Hunucmá. | Abandoned quarry flooded with water from a cenote and surrounded by native vegetation. |
| 7. El Islote. 21° 28.656 N, -88° 39.975 W. 25 km NE of Dzilam. Dzilam de Bravo State Reserve. | El Islote is located between the coastal lagoon and the eastern coast of the Dzilam de Bravo State Reserve, close to a place called “Las Bocas”, characterized by extensive sandy areas devoid of vegetation and with mangrove trees bordering the lagoon. |
Cenote Tunkás (site 2). With a total effort of 2.3 km, we surveyed the perimeter of an open cenote with a slope of approximately 7 m from the upper edge to the water body. The vegetation in Cenote Tunkás was tropical deciduous forest with petén along the shore and aquatic vegetation with dense riparian vegetation in a gallery forest composed by Metopium brownei, Bursera simaruba, Phitecelobium albicans, Thrinax radiata, and Nymphaea ampla, to mention some of the species that provided shade to the cenote.
In the bank, paths leading from the cenote to an otter’s burrow were found devoid of vegetation and the path edge vegetation flattened at ground level, which indicates constant use by otters. Two burrows were found near the water's edge, outside one of them tracks were found; an annal gland jelly mark was found on top of a rock in the middle of the slope near one of the burrows (Figure 2). During the survey the high-pitched bark of an otter was heard, and the recording was reproduced to entice the otter to answer the calls, which the otter did.

Figure 2 Records of Lontra longicaudis in Yucatán. A) Tracks at San Crisanto. B) and C) Den and otter trail from the water to the den at Cenote Tunkás. D) Latrine at Yalahau Lagoons State Park (Parque Estatal Lagunas de Yalahau). E) A spraint dispersed by recent rain on a trunk at Chichankanab Lagoon, Quintana Roo (border with Yucatán). Note the presence of fish scales and fresh remains of water crab. F) Footprint at Cocodrilo Lagoon near Sisal. G) Footprint at San Antonio Chel. H) Footprint at El Islote near Dzilam de Bravo.
Yalahau Lagoons State Park (site 3). This site was visited during the month of October for four years (2017 to 2019 and 2021). The survey conducted in 2017 included a lagoon’s bank section, with a total effort of 2.1 km. The following years the area surveyed was increased to include the total perimeter of the lagoon of 8 km. The vegetation at Yalahau Lagoon was composed by popal and tropical deciduous forest subjected to annual floods, with species such as Haematoxylum campechianum, Dalbergia glabra, and Mimosa bahamensis. As evidence, an otter spraint was collected from a rocky outcrop among grasses in the lagoon’s bank, even though the water level was overflowed due to the extra seasonal rains.
During the 2018 survey, an otter latrine was found on a rock, in the northern portion of the lagoon (Figure 2). The islands of hydrophilic vegetation that are near the northern portion of the body of water were also visited, during this activity we heard an otter’s bark, thus we reproduced the zoo’s vocalization recording to incite the otter to answer the call to confirm the presence of the species; then, the individual came close enough to obtain a visual record. For the 2019 survey, the latrine was abandoned probably due to the overflow of the lagoon’s water level, as many areas of riparian vegetation.
In 2020, due to the COVID 19 pandemic, no surveys were carried out because the lagoon’s state park was closed during the contingency. Activities resumed in October 2021, when indirect records of the species were found in the northern portion of the water body, a site close to where we observed an otter in 2018. In 2021 we also surveyed other two smaller lagoons close to the main one and in all of them we found indirect evidence of otter’s presence consisting in tracks and feeding sites.
Chichankanab Lagoon (site 4). We covered 4.8 km on canoe and 1.7 km on foot. The vegetation at the western bank was tropical deciduous forest with popal. The tropical forest species at the eastern bank were Lonchocarpus castilloi, Alseis yucatanensis, Brosimum alicastrum, M. zapota, Ceiba pentandra, and H. campechianum, and banks were surrounded by red mangrove (R. mangle; Merediz-Alonso 2004). Three places with abundant otter signs consisting of feces on the roots and branches of mangrove trees were found on the banks of the lagoon. However, many of these were washed away after the rain that occurred that same day.
Sisal (site 5). We surveyed 10 km of coastal lagoons and mangrove forest at coastal areas in the El Palmar State Ecological Reserve. The vegetation was dominated by coastal dune bushes and mangrove, with Suaeda linearis, Lycium carolinianum, Tribulus cistoides, Canavalia rosea, Euphorbia mesembrianthemifolia, Ernodea littoralis, Ipomea pes caprae, Coccoloba uvifera, R. mangle, and T. radiata. Neotropical otter footprints were found over a sand-gravel substrate near Cocodrilo Lagoon at low tide.
San Antonio Chel (site 6). The survey consisted of 1.5 km on an abandoned limestone quarry with an excavated pit filled with water. The surrounding area included a flat section of fine gravel of about 3,000 m2 with vegetation corresponding to floodplain forest with H. campechianum, Cameraria latifolia, M. brownei, M. zapota, B. simaruba, C. aesculifolia, Cochlospermun vitifolium, button mangrove (Conocarpus erecta), D. glabra, Neotropical otter footprints and tracks were found over a sand and gravel substrate near the pit.
El Islote (site 7). In October 2019, 10 km were surveyed in mangrove and beach sandbars areas near the coast of Dzilam de Bravo State Reserve. The area was mostly devoid of vegetation; however, it covered part of the mangrove forest in the swamp. The vegetation included red mangrove, button mangrove, and black mangrove (Avicennia germinans), some dune vegetation with sea grape or uvero de playa (C. uvifera) and grasses. We found indirect signs of otters consisting of footprints on a humid sandy substrate between the coastal lagoon’s shore and coastal dunes. In October 2021, a combined boat and foot survey was done in the same area, on this occasion the tide was high, and the cenotes inside the mangrove forest were also overflood due to extra-seasonal rains, therefore we found no evidence of otters on the mangrove branches and roots.
Discussion
According to the potential distribution model, the available habitat for the otter were generally located in the coastal lagoons in the western and northern coasts of Yucatán, and in a geological structure named "Ring of Cenotes" which are the remnants of the meteorite that impacted the area of northern Yucatán at Chicxulub ca 66 Myr and produced thousands of cenotes and other geological features (Connors et al. 1996). The “Ring of Cenotes” probably connects suitable habitat with many cenotes of several types including open cenotes which are nearby, and land depressions subject to flood during rainy season which form temporal lagoons (Aguilar et al. 2016).
Direct and indirect evidence of otter’s presence was found in seven verified sites predicted with habitat suitability by the potential distribution model. These sites showed availability of prey species, including crustaceans, fish, reptiles, and birds, which are the main food sources for otters, as well as adequate vegetation cover, density and interconnectivity between land, water, and freshwater sources; these two aspects are essential for the survival of otters.
Since otter’s presence was confirmed in mangrove forests, epicontinental lagoons and cenotes, our results were consistent with previous studies on Neotropical otter ecology performed in other regions of Mexico (Macías-Sánchez 2003; Mayagoitia-González et al. 2012; Hernández-Romero 2016).
We report the first records of Neotropical otter in different inland areas of the Yucatán peninsula and noteworthy direct observations and the second record of Neotropical otter in the coastal locality of San Crisanto after a 20-year span (Sánchez and Gallo-Reynoso 2007). Otter’s presence was corroborated in Ría Celestún after an 18-year span, and new coastal records were found at Sisal, Dzilam de Bravo (El Islote), as well as new records at Chichankanab epicontinental lagoon.
The Chichankanab Lagoon is probably connected to the hydrological basin of the State of Quintana Roo; this basin includes several lagoon systems connected to Laguna de Bacalar, where a Neotropical otter was filmed in April 2020 (Hernandez 2020), and Laguna de los Milagros where otters have been previously recorded (Gallo-Reynoso 1997; Gallo-Reynoso and Meiners 2018).
Outstandingly, at San Crisanto marshes, the cenote is located among salt-extraction parcels, which neutralize the high concentration of dissolved salts in the area and made it suitable for otters since they can tolerate salinity levels of 2 to 3 ppm; the same condition was recorded at Celestún, Sisal, and Dzilam de Bravo (El Islote).
The marking activity of the Neotropical otter is known to decrease during the rainy season, due to the flooding of latrine areas (Gallo-Reynoso et al. 2016). We visited Yalahau Lagoons State Park when supposedly the rainy season would be over by October, but we found high-water level resulting from heavy rains of an extended rainy season during 2017 to 2019 and 2021 surveys; nevertheless, we found an active latrine in 2018. These rains overflood the rocks, branches, roots and fallen trunks, thus washing away any spraint residues on latrine-suitable areas.
Our results pointed out that the Neotropical otter is associated with large freshwater bodies such as open cenotes, aguadas, epicontinental lagoons, coastal lagoons, and marshes in Yucatán which are major geological features of the state (Connors et al. 1996; Aguilar et al. 2016). It is important to continue searching for otters in the central areas of the Yucatan peninsula to unravel possible mobility between sites occupied by otters. The potential distribution model suggested habitat availability at many nearby locations, even though the lack of runoff waters and rivers hinders otter’s mobility. Therefore, it remains unclear if otter populations might be marginal (i. e., isolated) because the connectivity between these available habitats for otters is unknown, although we found otters distributed across the Ring of Cenotes and in other areas of Yucatán which points out to the presence of a certain connectivity due to geological features (Aguilar et al. 2016), that should be thoroughly investigated.
The distribution of the Neotropical otter in Yucatán is poorly known in the System of Natural Protected Areas of Yucatán (SANPY) and currently lacks a management plan or a specific conservation strategy for the species (Gallo-Reynoso 2013; Sosa-Escalante et al. 2013). The results of our research can be used to support the prioritization of sites for the conservation of the Neotropical otter, such as the Yalahau Lagoons State Park and the Ring of Cenotes and Chichankanab lagoon.
Conservation implications. The current growth and expansion of human populations and related urbanization generates a high demand for subsurface freshwater, along with groundwater pollution due to organic and solid wastes, agricultural and urban wastewater; all of which drain to surface and subsurface waters and pose a major threat for the Neotropical otter and its preys in the studied area (Arcega-Cabrera et al. 2014; Polanco-Rodríguez et al. 2015).
In areas of environmental suitability for Neotropical otter’s presence, we suggest organizing workshops to raise awareness on the biological importance and environmental services provided by this species in the different ecosystems, which might be translated into community strategies for habitat and water quality conservation (Hernández-Romero 2016). Otter populations have successfully recovered in several parts of the world thanks to legal protection programs and strict legislation addressing pollution of water bodies (Carone et al. 2014; Rheingantz et al. 2021), according to international instruments acknowledged by Mexico (Agenda 21, Río 1992).
It is recommended that the natural protected areas of Yucatán (Sisal, Yalahau Epicontinental Lagoons and Dzilam among others), and Federal protected areas such as Ria Celestún Biosphere reserve, where we found Neotropical otter presence, be acknowledged as refuge areas for their protection based on the Mexican General Law for Wildlife (Ley General de Vida Silvestre), and the Biosphere Reserve Ria Lagartos should be examined for Neotropical otter’s presence.











 nueva página del texto (beta)
nueva página del texto (beta)


