Introduction
Scavenging is a mode of feeding in which organisms acquire nutrients from carrion. Obligate scavengers, such as vultures, rely entirely on carrion as a food resource, whereas facultative scavengers acquire some, but not all of their nutritional needs from carrion. Scavenging is phylogenetically widespread in vertebrates and invertebrates, and plays an essential role in terrestrial ecosystems. For instance, this mode of feeding is crucial for the recycling of energy and matter in food webs, and for accelerating nutrient cycling and widely distributing these nutrients across the landscape (Putman 1978; Braack 1987; DeVault et al.2003; Selva and Fortuna 2007; Parmenter and MacMahon 2009; Bartonet al.2013). In addition, scavenging can have extensive consequences for the shape and stability of food webs (Wilson and Wolkovich 2011; Beasleyet al.2015), and have far-reaching effects on organisms and populations, including shaping the evolution of behavior, social systems, and inter- and intra-specific interactions (Cooper 1991; Shivik 2006; Krofelet al.2012; Moleón et al. 2014; Allen et al. 2015).
Due to its nutrient-rich, yet spatially and temporally patchy distribution, carrion is a unique resource that can have important effects on soils (Bump et al. 2009), microbes (Yang 2004), plants (Towne 2000; Bump et al.2009), trophic webs (Bartonet al.2013), nutrient cycling and species diversity (Hocking and Reynolds 2011; Olsonet al.2012; Bartonet al.2013). Large and small-scale habitat differences can affect the fate of carrion, and ultimately its availability for, and monopolization by scavengers (DeVault and Rhodes 2002; DeVault et al. 2004; DeVault et al. 2011; Turner et al. 2017; Pardo-Barquín et al. 2019; Stiegleret al.2020). For example, Houston (1985) found that carrion persisted longer for vertebrate scavengers in Neotropical rainforests compared to Afrotropical forests. Factors such as temperature, humidity, rainfall, season, and the composition of the insect community can affect the rates at which decomposers utilize carcasses (Houston 1985; DeVault et al. 2003; Selva et al. 2005; Selva and Fortuna 2007), and thus the availability of this food source to scavengers (DeVault et al. 2003).
Carrion availability can vary greatly within and across terrestrial ecosystems, and depends on the cause of mortality and the accessibility of the carcass location (Moleón et al. 2019). Empirical data showcasing the prevalence of carrion biomass in different ecosystems are scarce (DeVault et al. 2003; Barton et al. 2019; Moleón et al. 2019). The proportion of mortality due to predation versus other causes probably results in an important amount of food for scavengers, making it likely that more energy is transferred through scavenging than predation in trophic webs (Wilson and Wolkovich 2011). The percentage of animal deaths due to causes other than predation is thought to be fairly high in many ecosystems: >95 % of reindeer deaths in northern Scandinavia (Tyler and Øristland 1995), 25 to 88 % for large mammals in a Polish forest (Jedrzejewski et al. 1993), ~70 % for large ungulates in the African savannah (Houston 1979).
However, the availability and utilization of small-mammal carcasses is inherently more difficult to determine. Due to their size, small-mammal carrion can be consumed entirely by a scavenger and disappear more quickly. Small-mammal carrion may be more difficult to detect (by scavengers or researchers), especially in a structurally-complex habitat. Some estimates calculate that ~40 % of small-mammal mortality is made available to scavengers and decomposers (Akopyan (1953) as referenced by Putman (1976) and DeVault et al. (2003). Oksanen et al. (1997) showed 83 to 98 % of small-mammal deaths in the Arctic were not due to predation. Undoubtedly, the large reproductive output of most small mammals likely provides a large number of carcasses (Cowles and Phelan 1958). However, this does not show the importance of the energetic link between small-mammal populations to the scavenger community.
Even though scavenging is a widespread and important ecological process, it is poorly understood, underestimated, or overlooked in food web models (Wilson and Wolkovich 2011; Barton et al. 2013, Moleón et al. 2014), stemming in part by the difficulty in quantifying carrion in ecosystems (Barton et al. 2019; Moleón et al. 2020). Additionally, the role of scavenging in ecosystems has been oversimplified, with facultative scavenging often categorized as random or opportunistic, although research is now showing highly nested patterns and complex interactions dictating scavenger community structure (Selva and Fortuna 2007; Olson et al. 2016). In particular, we lack a comprehensive understanding of the fundamental role of scavenging in various ecosystems (Beasley et al. 2019), and the factors that shape and structure scavenging communities and scavenger behavior (DeVault et al. 2003; Wilson and Wolkovich 2011; Barton et al. 2013). For instance, despite facultative scavengers being more common than obligate scavengers, they are neglected in trophic studies. This is due, in part, to facultative scavengers described typically as predators or omnivores, and their roles as scavengers being unknown or ignored (Wilson and Wolkovich 2011). Scavenging by vertebrates is underestimated (Wilson and Wolkovich 2011; Barton et al. 2013), as scavenging research has often focused on arthropod scavenging (Barton et al. 2013). Additionally, research on understanding the species diversity and composition of the scavenger communities is timely, as they have been negatively affected by global change (Olson et al. 2012; Beasley et al. 2015; Buechley and Şekercioğlu 2016), and anthropogenic influences can drive changes in scavenger assemblages (Sebastián-González et al. 2019; Sebastián-González et al. 2020). Therefore, in order to better model food web dynamics, understand the intricate ecology of terrestrial ecosystems, and restore ecosystem services, it is important for scavenging to be quantified, and for scavengers to be identified.
Studies focused on elucidating the role and patterns of scavenging in Neotropical ecosystems are also lacking (Beasley et al. 2019; Sebastián-González et al. 2019). This is particularly true for understanding the dynamics and contribution of vertebrate scavengers and small-mammal carrion in these complex ecosystems. Studies that investigate scavenging in the Neotropics have focused mostly on arthropods or vultures, or have detailed scavenging observations of single species (Houston 1985; Houston 1986; Houston 1988; Lemon 1991; Gomezet al.1994; O’Donnell 1995; Villegas-Patracaet al.2012; Mallonet al.2013; dos Santos et al. 2014; Arroyo-Arce et al. 2016; Ucha and Santos 2017; Romeroet al. 2020). Scavenging is thought to be significant in Neotropical forests. Houston (1986) estimated in Barro Colorado Island, Panama, that 4.1 kg/km2 of mammals die every day. This amount surpasses those calculated for Afrotropical forests: 4.3 times higher than Lombe Forest, Cameroon and 1.58 times higher than Kibale Forest, Uganda (Houston 1985). Even though some proportion of these deaths is due to predation (Houston 1985) a large amount of carcasses would be made directly available to the scavenger community.
The goals of this project are to understand the importance of small-mammal carrion, scavenging, and factors that affect scavenging rates in a mid-elevation Neotropical rainforest. Specifically, I aim to study: 1) What are the rates of small-mammal carcass removal? 2) How does visual conspicuousness (position in the leaf litter) and size of the carcass affect scavenging rate? 3) What vertebrates are involved in scavenging the small-mammal carcasses? I use an experimental approach to explore these questions by placing fresh rodent carcasses above and below the leaf litter, along with trail cameras. This project provides insight into the importance of small-mammal carrion as part of the food-web dynamics of mid-elevation tropical rainforests.
Materials and methods
I conducted this study in Las Brisas Nature Reserve (10.0670°, -83.6376°), Limón province, Costa Rica. Las Brisas is situated in the northeastern slopes of the Volcán Turrialba, within the central volcanic range of Costa Rica. The elevation in the reserve is 650 - 1,030 m above sea level, situating it as a mid-elevation forest. Las Brisas is composed of a mixture of old growth and secondary forests of various stages, along with some scrub and open areas. The reserve is located on a continuous range from the Caribbean lowlands to the highlands of Volcán Turrialba (https://www.lasbrisasreserve.com/aboutus). Access to the reserve is limited because it is privately owned and maintained, and although there is a system of trails, human presence is relatively limited.
To assess scavenging rates I set out and surveyed 194 mouse carcasses (Mus musculus) within the forested area of the Las Brisas Nature Reserve from 29 May through 8 June 2018. I purchased commercially available euthanized and frozen feeder mice (mouse meals for pets such as snakes) from a local supplier. Mouse carcasses were thawed at room temperature approximately 1 to 2 hours prior to placement in the field. I handled all carcasses with latex gloves, and weighed the thawed carcasses before deploying them. The average carcass weight was 11.64 g (n = 194; SE = 0.40; SD = 5.55; median = 10.5). The weight ranged from 3.4 to 25.8 g. The carcasses had white pelage, which was the only mouse color available from the supplier, but also mimics the ventral pelage of several species found in these Costa Rican forests.
I placed the carcasses ventral side up in areas that were accessible by the trail system in Las Brisas. Carcasses were placed at a minimum of 50 m from each other, 0 to 5 m off the trails. To test how visual conspicuousness affects scavenging rates, I placed 98 carcasses on top of the leaf litter and 96 below the leaf litter. To conceal the carcasses below the leaf litter, I moved fallen leaves with a stick, placed the carcass on the ground and covered it fully with the displaced leaves. After deploying the carcasses, I surveyed them daily until they had disappeared or had decomposed past the point of having any flesh remaining, which is how I determined carcass removal time (days elapsed since carcass deployment) since trail cameras are not triggered by invertebrates.
In addition to the daily sampling, I also placed trail cameras on each deployed mouse carcass to document scavengers. I used Ltl Acorn 5210-A (Guangdong, China) and Foxelli Outdoor Gear Oak’s Eye Trail Camera (Vlaardingen, The Netherlands), on the most sensitive triggering settings, to record video for 30 seconds with no lapse time between videos. Trail cameras were set to trigger based on motion. The cameras were placed on existing structures within the forest a few meters away from the deployed carcass to identify scavengers. Although I attempted to set a camera on each deployed carcass, human error or equipment malfunction allowed me to get video recordings from 160 deployments. When reviewing the videos, I was conservative when identifying scavengers; animals were only categorized as scavengers if they were seen grabbing or consuming the carcass on the video.
Throughout the project I noticed that small mounds appeared where many carcasses had been deployed, and it was not until later in the experiment that I realized that something too small to be picked up by the trail cameras was burying the mouse carcasses. To investigate what was doing this, I dug below a few of the mounds and was able to follow narrow tunnels to my deployed carcasses buried ~15 to 20cm deep. Attached to one of the buried mice were two Coprophanaeus corythus (Scarabaeidae) beetles. Unfortunately, I did not start recording data on whether beetles were burying the carcasses until too late into the study, but a large proportion of my carcass deployments exhibited the unique dirt mounds.
I used a general linear model to test the effect of weight (continuous independent variable) and position on the leaf litter (categorical independent variable - above or below) on carcass removal time (dependent variable - transformed data on days elapsed since carcass deployment). I transformed the dependent variable of days elapsed since deployment to eliminate heteroskedasticity by taking the natural logarithm of the values plus one. I calculated carrion consumption rate, a measure of carrion biomass consumed divided by consumption time (g/hr), for carcasses in the two leaf litter treatments (above and below), and all data combined. I used MiniTab v. 18 for all statistical analyses. This study was approved by the University of Wisconsin’s IACUC committee and Las Brisas Nature Reserve management, and permits were issued by Costa Rica’s government agency MINAE.
Results
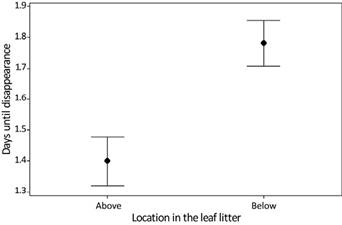
Scavengers removed the majority of mouse carcasses throughout the study. Of 194 carcasses placed in the forest, 193 (99.48 %) were removed by vertebrate or invertebrate scavengers, and only one was fully consumed by decomposers. Although I did not quantify the efficiency of beetles in burying carcasses, their ubiquitous mounds suggest they were dominant in monopolizing this resource. Only one carcass, placed below the leaf litter, decomposed and left behind remains of fur and bones six days after deployment. When all data are pooled together, the average number of days for carcass removal by scavengers is 1.57 days (SE = 0.05; SD = 0.71; median = 1). Over half (54.12 %) of the mouse carcasses were removed within 24 hours, and 90.21 % of carcasses were removed within 48 hours (Figure 1). The average consumption rate was higher for carcasses placed above ground (0.396 g/hr; SE = 0.022; SD = 0.221) than those placed below the leaf litter (0.306 g/hr; SE = 0.017; SD = 0.174); overall carrion consumption rate was 0.352 g/hr (SE = 0.014; SD = 0.204).
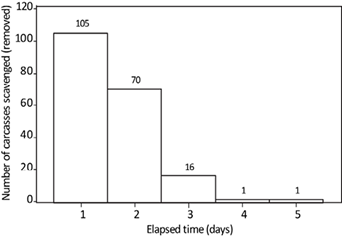
Figure 1 Histogram of number of carcasses removed by days after carcass deployment using all data pooled together.
The general linear model, with time to carcass removal as the dependent variable, shows a significant difference for both independent variables: weight (n = 194; F = 8.67; P = 0.004), and position on the leaf litter (n = 194; F = 19.58; P < 0.001; Figure 2). The model summary shows an R-squared of 12.77 %. Weight had a small significant effect (regression slope for above and below leaf litter = 0.01521) resulting in larger carcasses lasting slightly longer. Overall, the carcasses placed above the leaf litter are removed more quickly than those below the leaf litter. The average number of days until the removal of a carcass placed above the leaf litter is 1.35 days (n = 97; SE = 0.06; SD = 0.63; median = 1). For these carcasses, 70.1 % were removed by 24 hours, and 96.9 % by 48 hours (Figure 3). The maximum number of days before a scavenging event for carcasses above the leaf litter is five days. In contrast, the average number of days until the removal of a carcass placed below the leaf litter is 1.78 days (n = 96, SE = 0.07; SD = 0.73; median = 2). Removal rate for carcasses placed below the leaf litter is 38.5 % within 24 hours, and 83.5 % within 48 hours. The maximum number of days before scavenging for carcasses under the leaf litter is 4 (Figure 4). The regression equation for carcasses placed above the leaf litter is Ln (days until removal + 1) = 0.0631 + 0.01521 × weight. The regression equation for carcasses placed below the leaf litter is Ln (days until removal + 1) = 0.3163 + 0.01521 × weight.
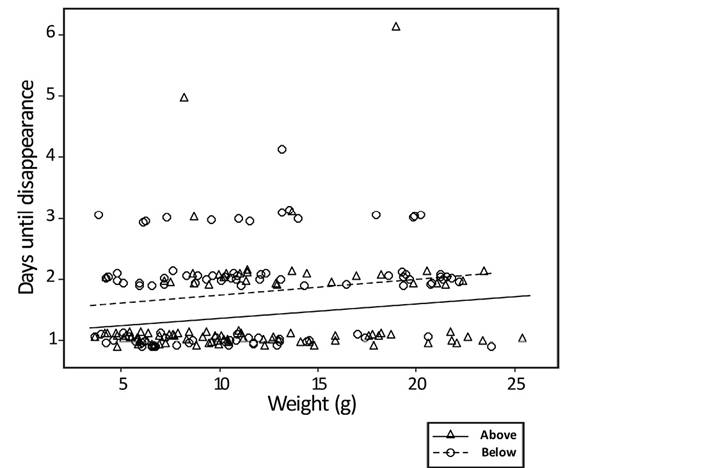
Figure 2 Scatterplot (with regression and position in the leaf litter) of days until disappearance (how long it took for a scavenger to remove the carcass) vs. weight of mouse carcass.
Out of the videos captured on 160 carcass deployments, 10 videos showed a scavenging event by a vertebrate (6.25 %). The most common vertebrate scavenger recorded was the common opossum (Didelphis marsupialis), followed by gray four-eyed opossum (Philander opossum) and coyote (Canis latrans). Four videos showed a Russet-naped Wood-Rail (Aramides albiventris) pecking at carcasses, but not consuming the entire carcass. In five videos a variety of vertebrates are seen exploring and sniffing intently the area where the carcass was located, or had been located prior to disappearance. These videos showed tayras (Eira barbara), coatis (Nasua narica), and Russet-naped Wood-Rails interacting with the carcass or sniffing and searching where the carcass had been deployed (Table 1).
Discussion
Small-mammal carcasses were removed very quickly in this study (1.57 days on average), and only one carcass decomposed in place. The proportion of carcasses removed in the first days, show that carrion is an important and sought-after resource in these Neotropical rainforests. Indeed, the time to carcass removal was assuredly overestimated since the presence/absence of the carcass was determined via surveys that were only conducted every 24 hours. The speed at which carrion is monopolized can vary by habitat type and ecosystems (Beasley et al. 2015); studies in temperate regions show a wide range of carcass disappearance speed for small-mammal carrion, but are typically longer than that reported here: 2.58, 5.6, and 1.23-3.30 days (DeVault and Rhodes 2002; DeVault et al. 2004; Olsonet al.2012). Research done in the Neotropics with domestic chickens found a quick carcass removal time of ~10 hours (Houston 1986; Houston 1988). Temperature and humidity in the tropics likely create conditions in which microbes colonize carcasses quickly, and olfactory cues that can alert scavengers of carrion are emitted more quickly. Competition between a wide range of scavengers and decomposers is seemingly high.
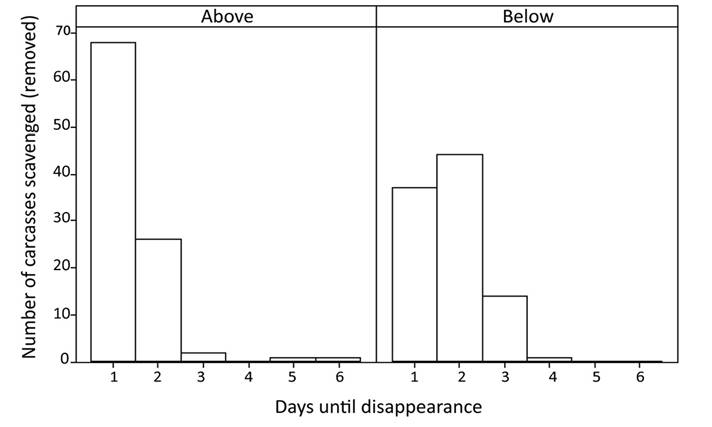
Figure 4 Histograms of number of carcasses removed by days after carcass deployment for above and below the leaf litter.
One of the most striking aspects of this study is the relatively few instances of recorded scavenging by vertebrates. My study’s rate of efficiency by vertebrate scavengers (6.25 %) is much lower than those reported in the literature. Although differences exist based on the location of the study, and the type and size of carcass used, published estimates of vertebrate scavenging efficiency in terrestrial habitats averages ~75 %, and ranges from 13 % to 100 % (DeVault et al.2003). If we focus on research that only utilizes small mammals as carrion bait, vertebrates are also the dominant scavengers, ranging in efficiency from 35 % to 100 % (Mullen and Pitelka 1972; DeVault and Rhodes 2002; DeVault et al. 2003; DeVault et al. 2004; DeVault et al. 2011; Turner et al. 2017). Given the large number of deployments that had working trail cameras during pickup, equipment malfunction is unlikely to be the reason why we see this trend. It is, however, probable that the small-mammal carcasses were consumed or removed by invertebrates too small to activate the camera.
Invertebrate scavengers can consume high proportions of carcasses compared to vertebrates (Cornaby 1974; Ray 2014), and beetles were likely a strong force in the removal of whole carcasses during this study. Although I did not quantify the removal of carcasses by beetles from the start of the study, I did notice many conspicuous mounds in the carcass deployment locations. Similar research in a Costa Rican lowland forest showed that ~70 % of small-mammal carcasses deployed on the ground were removed by beetles (Romero, unpublished data).
Table 1 Vertebrate scavengers and their activity at carcass location.
| Common name | Species | Number seen | Interaction with carcass |
|---|---|---|---|
| Common opossum | Didelphis marsupialis | 6 | Scavenging |
| Russet-naped Wood-Rail | Aramides albiventris | 4 | Pecking at carcass, but not consuming or removing it |
| White-nosed coati | Nasua narica | 3 | Sniffing near carcass or where carcass had been located |
| Coyote | Canis latrans | 2 | Scavenging |
| Gray four-eyed opossum | Philander opossum | 2 | Scavenging |
| Tayra | Eira barbara | 2 | Sniffing near carcass or where carcass had been located |
The results of this study pertaining to carcass weight are also probably a function of the interaction between the beetles and the carcasses rather than the vertebrate scavengers. We know that for the biomass of small-mammal carcasses, Coprophanaeus beetles are more effective in quickly locating and hoarding this resource. Given the regression equations, the smallest deployed mouse (3.4 g) would have been consumed or buried on average 8.18 hours quicker than the largest mouse (25.8 g). Very little ecological and behavioral information is available for these beetles (Edmonds 2010), and it is not known how long they take to bury a mouse carcass. The Coprophanaeus corythus beetles I found were very small (~25 mm), so it is not hard to imagine that larger carrion would generally take longer to bury. At some point carcass size would become limiting to the beetles’ ability to bury and exploit carrion. There must be a threshold at which these beetles no longer bury carcasses, and vertebrate scavenging may become more dominant, which may result in carrion partitioning, a pattern documented in other scavenging systems (see Moleónet al. 2017; Muñoz-Lozano et al. 2019).
How scavengers detect and locate carrion may be critical to understanding how this system of scavengers is maintained. Some research supports the idea that visual conspicuousness is important for scavengers to locate carrion (Selvaet al. 2005). Olfactory cues, however, may be more important in attracting a suite of vertebrate scavengers to carcasses (Houston 1986; DeVault and Rhodes 2002; Potieret al. 2019). DeVault and Rhodes (2002) did not find a significant difference in the rates of scavenging on carcasses placed above and below leaf litter. Houston (1986) found that Neotropical vultures were able to locate the general area where chicken carcasses had been deployed above and below the leaf litter, and vultures were able to consume these carcasses within hours regardless of their leaf-litter position. My study found a difference in the time to removal of carcasses placed above and below the leaf litter, although the proportion of carcasses removed by scavengers (compared to entirely decomposing) was almost 100 % for both. In addition, it is important to note that while carcass time to removal was significantly different for those placed above and below the leaf litter, carrion in both categories were scavenged relatively quickly, and the difference equated, on average, to only a matter of hours (above = 1.35 days, below = 1.78 days). This result may be due to microhabitat differences in relation to temperature, humidity, and exposure to direct sunlight (Sayer 2006), which affects the rate at which carcasses decompose and emit odor (Putman 1978; Sheanet al.1993). As DeVault and Rhodes (2002) note, olfactory cues are likely important for mammalian scavengers; and both their study and mine recorded mammals sniffing in areas where carcasses had been placed but had already disappeared.
This study highlights the importance of small-mammal carrion as a sought-after resource in Neotropical rainforests. Carcass consumption rate may seem low (cf.Sebastián-González et al. 2016; Gutiérrez-Cánovas et al. 2020; Sebastián-González et al. 2020), but is most likely a function of the small carcass size. The speed at which small-mammal carcasses are entirely consumed or hoarded by scavengers is extremely fast. The relatively low rate of vertebrate scavenger efficiency in exploiting small-mammal carrion makes this ecosystem particularly unique. Further research should explore why beetles are seemingly outcompeting vertebrates. They may be locating these carcasses faster, detecting putrifaction cues at lower concentrations, or are simply highly abundant and able to arrive at the carcasses more quickly. While the efficiency of different scavenger guilds (vertebrates vs. beetles) can vary in different habitats, ecosystem function is typically sustained (Sugiuraet al.2013; Sugiura and Hayashi 2018). Therefore, research focused on understanding how these complex interactions between invertebrate and vertebrate scavengers maintain ecosystem function would be timely to help create a more developed framework for Neotropical food webs.











 nueva página del texto (beta)
nueva página del texto (beta)