INTRODUCTION
In the aquatic ecosystems, primary producers can be the main route of metals to consumers of the following trophic levels, including humans (Castañé et al. 2003). Phytoplankton can bioaccumulate metals as part of biogeochemical cycles (Sánchez-Marín et al. 2014). However, algae may show a higher intracellular concentration of metals than the environment around them. These metals are potentially toxic, likely to be biomagnified in aquatic systems due to their persistence despite being present in small concentrations (Kumar et al. 2015). Metals such as Hg, As, Cr, Pb, Cu, Cd, Ni, Co, and Zn are considered toxicologically important (Castañé et al. 2003, Kumar et al. 2015). One of the principal pollutants present in aquatic environments due to natural and anthropogenic processes is lead (Alvarado-Flores et al. 2012, Sánchez-Marín et al. 2014, Urien et al. 2015). Lead has no vital role in biological processes and it is toxic at low concentrations (Kumar et al. 2015, Urien et al. 2015, Hernández-Flores et al. 2020). It can cause irreversible health effects, affecting the central nervous, hematopoietic, renal, and reproductive systems, as well as the liver (Rubio et al. 2004, Flora et al. 2012). Lead is considered by the US Environmental Protection Agency (USEPA) as one of 129 priority pollutants, and it is number two on the Agency for Toxic Substances and Disease Registry (ATSDR) 2005 list (Rubio-Franchini et al. 2016). Also, Pb is one of the six global pollutants in The world’s worst pollution problems 2016 report (PEGC 2016). The degree of bioaccumulation of Pb in aquatic organisms depends on the dynamics of the trophic network, availability, persistence, and physical and chemical characteristics of toxic substances in water (Rand and Petrocelli 1985, Rubio-Franchini and Rico-Martínez 2011). Also, the entry of metals into the cell can occur when they are in ionized form through transport systems that use other physiologically important cations such as Ca2+, Mg2+, Cu2+, and Zn2+ (Castañé et al. 2003, Rubio-Franchini and Rico-Martínez 2011, Lavoie et al. 2013, Sánchez-Marín et al. 2014). Bioaccumulation occurs when the ingestion rates exceed the excretion rate (Rubio-Franchini and Rico-Martínez 2011, Urien et al. 2015). The bioconcentration factor (BCF) is the ratio of a chemical’s concentration in an organism divided by the concentration of the same chemical in the environment. In contrast, the bioaccumulation factor (BAF) is the proportion of a chemical’s concentration in an organism divided by the concentration of the same chemical resulting from any route of exposure such as diet, ingested water, or transport across a surface (Walker et al. 2006, DeForest et al. 2007, Zhang et al. 2016). The diet is the main route for the entry, transfer, and accumulation of metals in aquatic food webs, being of ecological importance (Soto-Jiménez et al. 2010, Soto-Jiménez 2011).
Lead bioaccumulation has been reported in aquatic organisms of different trophic levels with BAF values from 6413 in Oscillatoria sp. up to 15 000 and 70 789 in Moina micrura and Simochephalus vetulus, as well as values of 90 263-1 877 205 for Hyalella azteca and Culex sp. (Rubio-Franchini and Rico-Martínez 2011). Similarly, Zhang et al. (2016) recorded concentrations of lead in water (5.5 µg/L), sediments (57.46 mg/kg), and aquatic organisms with BCF of 35.85 for phytoplankton, and BAF values of 2.89, 1.24, 1.09, and 0.47 for zooplankton, fishes, shellfishes, and shrimps, respectively. Soto-Jiménez et al. (2011) evaluated the exposure to sublethal doses of lead in the microalgae Tetraselmis suecica under laboratory conditions, reporting an increase in BCF from 930 to 3630, while the concentration of the metal decreased towards the higher trophic levels.
Lead accumulation has also been reported in aquatic organisms of commercial importance for human consumption. Bivalve mollusks such as the mussel Lamellidens marginalis accumulate 0.12-1.20 mg/kg (Kumar et al. 2017), the oysters Crassostrea corteziensis and Crassostrea virginica accumulate 1.11 µg/g (Páez-Osuna and Osuna-Martínez 2015) and up to 26.32 µg/g (Vázquez-Botello et al. 2004), respectively. Even the clam Dosinia exoleta accumulates lead exceeding the European limits for human consumption (Darriba and Sánchez-Marin 2013). Similarly, crustaceans can accumulate lead. The shrimp Litopenaeus vannamei exhibits a low excretion rate, with little bioaccumulation in muscle, but greater accumulation in the head, gills, hepatopancreas, and exoskeleton (Osuna et al. 2014). Likewise, Abdennour et al. (2000) recorded maximum concentrations of lead in several prawns such as Atyaephyra desmaresti (11.5 µg/g), Palaemonetes varians (8.4 µg/g), Parapenaeus longirostris (2.3 µg/g), and Aristeus antennatus (1.69 µg/g).
Therefore, there is a risk to human health from consuming aquatic organisms contaminated with potentially toxic elements such as lead, which can be incorporated through trophic chains. In addition, potentially toxic elements might alter the structure and functioning of the aquatic community in a polluted ecosystem. It is essential to know the concentration at which lead accumulates in primary producers, its transfer through the digestive pathway, and its accumulation in organisms of higher trophic levels. Laboratory experiments with concrete and representative food chains are useful tools to understand the transfer processes of potentially toxic elements, offering the understanding of mechanisms involved in the bioconcentration, bioaccumulation, and biomagnification factors (Soto-Jiménez 2010). This study aims to assess the toxicity, bioaccumulation, and transfer of lead under controlled laboratory conditions by estimating BCF and BAF values in organisms commonly used in aquaculture, such as the microalgae Nannochloropsis oculata and the rotifer Brachionus plicatilis, considered as live food to supply the Malaysian prawn larvae Macrobrachium rosenbergii.
MATERIALS AND METHODS
Culture of the organisms
N. oculata was obtained from the microalgae collection of the Centro de Investigación Científica y de Educación Superior de Ensenada (CICESE). Its cultivation was carried out in Guillard f/2 medium (Stein 1979) with slight modifications (Pérez-Legaspi et al. 2018), at 18 ± 2 ºC under constant illumination (79.88 µmol/m/s) and aeration. Moreover, artificial seawater at 15 ‰ salinity was used as medium, which was prepared with distilled water (Felisa FE-390, < 1.5 ppm, pH 5.4-7.2, ~300 000 Ohm), and Instant Ocean synthetic sea salt [Na+, K+, Mg++, Ca++, Sr++, Cl-, SO4 - -, HCO3 -, Br--, B(OH)3, F-]. An algal sample from 5 days of cultivation was taken in the exponential phase to start with a concentration of 1 × 106 cell/mL for all treatments. We used the rotifer B. plicatilis strain Alvarado from the Alvarado Lagoon, Veracruz, Mexico as a primary consumer. This rotifer has been cultivated for more than 10 years in artificial seawater at 15 ‰, pH of 7.5, and temperature of 25 ± 2 ºC under continuous illumination at 27 µmol photons/m2/s, fed with N. oculata according to Moha-León et al. (2015) and Pérez-Legaspi et al. (2018). As a secondary consumer, 7-day-old M. rosenbergii Malaysian prawn larvae (zoea IV), obtained from a local aquaculture farm, which were kept in artificial seawater at 15 ‰, pH of 7.7, and temperature of 28 ± 2 ºC, with constant aeration supply.
Toxicity bioassays and sample process
The exposure of N. oculata to lead was carried out in accordance with the Selenastrum capricornutum growth test method 1003.0 of the USEPA (2002), with slight modifications (Pérez-Legaspi et al. 2016). Bioassays with the microalgae included negative control (non-toxic Guillard f/2 medium) and five lead concentrations (0.1, 0.5, 1.0, 5.0, and 10.0 mg/L) in triplicate. Standard solution of atomic absorption of Pb (NO3)2 from the highest purity available (J.T. Baker, Sigma, USA) was used for this study. The lead analysis was performed using atomic absorption spectrophotometry (AAS). The total volume of the test was 250 mL in each 500 mL glass flasks sealed with parafilm M film paper, keeping them in the same growing conditions for 144 h. The algal density was assessed at different exposure times (24, 48, 72, 96, and 144 h) in triplicate, using a Neubauer chamber cell counter (0.100 mm Loptik Labor) and an optical microscopy. Also, its dry weight was obtained according to the gravimetric method of Sorokin (1973) with slight modifications (Moha-León et al. 2018). The biomass samples were filtered through filter paper (55 mm diameter, Whatman GF/C Microfiber, retention nominal particle of 2.5 µm) and collected in a membrane. The biomass wet weight in the filter paper was registered using an analytical balance (Denver Instrument) and then placed into Petri dishes and dried in a culture oven (Ecoshel 9162) for 24 h at 35 ºC. The dry weight was estimated employing an analytical balance and a desiccator to evaluate the density of the filters plus the microalgal sample in triplicate, until a constant measurement is recorded and the biomass is determined by difference in weight. Also, microalgae samples (10 mL) were taken for each treatment and centrifuged (Cence H1650R) at 14 000 rev per 15 min at 4 ºC, eliminating the aqueous medium and recovering the cell pellet by rinsing it in 0.5 M ammonium formate (HCO2NH4) (Sigma-Aldrich, Switzerland). The salt was removed from the medium and the sample was centrifuged again, adding HNO3 instra with deionized water (1:1) (J.T. Baker, USA). After acid digestion, the concentration of lead was analyzed by AAS.
The rotifers were kept without food for 12 h before starting experiments in accordance to Sayegh et al. (2007) with slight modifications. Previously, the microalgae N. oculata was exposed to lead for 48 h at two concentrations, 0.1 and 1.0 mg/L. Bioassays began by placing 3000 rotifers collected directly by means of a transfer pipet and a stereoscope in 50 mL beakers containing the same culture medium at 15 ‰, with a density of 1 × 106 cell/mL of lead-exposed microalgae. These bioassays included a negative control (non-exposed microalgae) and two treatments with lead-exposed microalgae with each lead concentration per triplicate. Every 24 h, the rotifers were filtered with a mesh of 45 µm and transferred to a new test medium with lead-exposed microalgae. The bioassay was kept for 5 days at 25 ± 2 ºC with constant illumination. The lead concentration was analyzed by AAS in the rotifers (Rubio-Franchini et al. 2008), which includes 300 animals every 24 h per triplicate, transferring them to water deionized with HNO3 instra (1:1) in microcentrifuge tubes. Also, the dry weight of 100 rotifers was determined for each treatment.
For the lead accumulation assay with prawn larvae, 1000 rotifers fed 48 h before with lead-exposed microalgae (0.1 and 1.0 mg/L) were used. These rotifers were collected with a 45 µm mesh and rinsed with sterile artificial seawater at 15 ‰. Then, 200 mL of artificial seawater at 15 ‰ were added to fish tanks of 10 × 10 cm, and five larvae of prawns with 12 h of fasting were added to the tanks. The experiment was performed per quintuplicate and kept with constant aeration. The larvae were collected after 24 hours in test tubes. On the other bioassay, prawn larvae of the same stage were fed only with N. oculata (1 × 106 cell/mL) previously exposed to lead (0.1 and 1.0 mg/L) and collected after 24 h. HNO3 instra and deionized water (1:1) were added to the test tubes with larvae for acid digestion. After 24 h the samples were boiled gently for 30 to 60 min to dissolve the exoskeleton; subsequently, the lead analysis was performed by AAS.
Lead analysis
Lead determination was performed using AAS (Perkin-Elmer AAnalyst 800, detection limit of 0.005 µg/L) with graphite furnace and longitudinal Zeeman-effect background correction, according to the Mexican Official Standard for lead in water (NOM-117-SSA1-1994 [SSA 1995]), using a hollow cathode lamp. The calibration curves were maintained on the linear range with a coefficient of determination (R2) of 0.995 as well as less than 5 % variance between replicates. Internal quality control included a fortified reagent solution with 10 µg/L of lead nitrate in 2% HNO3 standard. Product No. 16595, Lot. BCBR7883V, ISO Guide 35 (99.9 %, Sigma-Aldrich, Switzerland), was used for analytical grade atomic absorption. The recovery rate of the reference sample was 80-120 %. Each sample was analyzed in duplicate. Deionized water was used for all analytical work. All materials used in this experiment were previously washed with 30 % nitric acid (J.T. Baker, USA) and rinsed with distilled water. Analytical spike recovery was 80-100 %. The relative percent difference between replicate analysis of subsamples was less than 10 %.
Data analysis
The BCF and BAF of lead were estimated in the microalgae, rotifers and prawn larvae, according to Paquin et al. (2003), Rubio-Franchini et al. (2008), 2016), and Alvarado-Flores et al. (2012), considering the following: BCF = lead concentration in the body (cell)/lead concentration in the environment; BAF = lead concentration in the body/lead concentration in food (or water ingested). Data analysis was performed using one-way ANOVA and post hoc comparison Tukey tests between treatments (p < 0.05) to estimate the value of No Observed Effect Concentration (NOEC), while confidence limits and R2 of the Effective Concentration where a significant effect is observed in 50 % of the exposed population (EC50) were estimated by simple linear regression analysis. Statistica v. 7.0 software (StatSoft 2004) was used for all statistical analyses.
RESULTS
The toxicity and adverse effect of lead on the population growth of N. oculata were confirmed by estimating the algal density and dry weight biomass of the microalgae exposed to the metal (Fig. 1, Table I). The adverse effect was detectable but insignificant (p > 0.05) after 24 h of exposure and significant when exposed to concentrations of 5 and 10 mg/L for 72 h (p < 0.05). Parameters evaluating lead toxicity in the microalgae are shown in Table II, where the NOEC value of 1 mg/L was obtained for 72 h. It is possible to observe that the EC50 value corresponds to 5.42 mg/L for 24 h, decreasing while increasing the exposure time, which suggests that from this concentration the growth rate of the microalgae is significantly inhibited (p < 0.05).
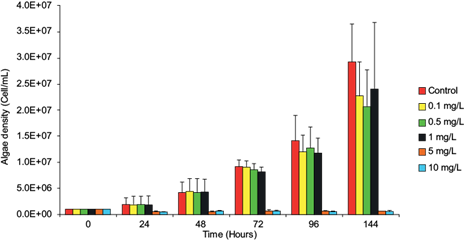
Fig. 1 Effect of lead on the cell density (cell/mL) of Nannochloropsis oculata. The bars correspond to the mean ± standard deviation (experiment by triplicate). Asterisks (*) denote statistical significance at p < 0.05.
TABLE I VALUES OF THE MICROALGAE AT DIFFERENT EXPOSURE TIMES FOR ALL TREATMENTS, AND FOR THE ROTIFER FED WITH MICROALGAE EXPOSED TO TWO LEAD CONCENTRATIONS (N = 100), BY TRIPLICATE.
| Pb (mg/L) | 24 h | 48 h | 72 h | 96 h | 144 h |
| Microalgae dry weight (µg) | |||||
| 0.1 | 29.6 ± 3.7 | 67.8 ± 2.8 | 137.5 ± 4.4 | 182.7 ± 0.2 | 392.7 ± 15.9 |
| 0.5 | 30.3 ± 1.2 | 59.6 ± 1.2 | 126.3 ± 1.2 | 181.5 ± 0.2 | 295 ± 11.3 |
| 1 | 23.3 ± 12.2 | 72.3 ± 2.8 | 124.0 ± 6.0 | 167.8 ± 2.3 | 356.1 ± 2.6 |
| 5 | 11.3 ± 0.1 | 19.4 ± 8.3 | 10.6 ± 0.1* | 10.6 ± 0.1* | 11.3 ± 1.9* |
| 10 | 9.6 ± 5.0 | 11.1 ± 0.9 | 11.4 ± 2.7* | 10.3 ± 1.4* | 9.4 ± 1.0* |
| Rotifer dry weight (µg) | |||||
| Negative control | 0.4 ± 0.09 | 0.9 ± 0.1 | 0.6 ± 0.5 | 0.2 ± 0.1 | |
| 0.1 | 0.6 ± 0.1 | 0.5 ± 0.2 | 0.4 ± 0.2 | 0.2 ± 0.1 | |
| 1 | 0.6 ± 0.4 | 0.3 ± 0.2* | 0.4 ± 0.2 | 0.1 ± 0.1 | |
*Significant (p < 0.05).
TABLE II ENDPOINT VALUES OBTAINED FROM CHRONIC TOXICITY TESTS FOR Nannochloropsis oculata EXPOSED TO LEAD AT DIFFERENT TIMES (mg/L).
| 24 h | 48 h | 72 h | 96 h | 144 h | |
| NOEC | - | - | 1 | 1 | 1 |
| EC50 | 5.42 | 4.56 | 4.04 | 3.67 | 3.25 |
| CL | 3.08-7.75 | 2.10-7.02 | 1.93-6.16 | 1.55-5.79 | 1.00-5.49 |
| R2 | 0.8356 | 0.7734 | 0.8099 | 0.7973 | 0.7613 |
EC50: effective concentration; NOEC: no observed effect concentration; CL: 95 % confidence limits; R2: correlation degree between microalgae growth and lead concentration.
Table I shows the dry weight of the rotifer B. plicatilis fed with the microalgae N. oculata previously exposed to lead. The results indicate that the dry weight of the rotifer decreases as the exposure time increases. In Table III, the lead analysis in the three organisms at each trophic level shows that this metal’s concentration in the microalgae increases as a response to the exposure concentration. The rotifer showed no signs of lead contamination when fed with N. oculata exposed to 0.1 mg/L of the metal; however, when the microalgae were exposed to 1 mg/L of lead, it was possible to detect a lead concentration of 13.47 µg/mg in the rotifer after 48 h of exposure. The prawn M. rosenbergii fed with N. oculata exposed to 1 mg/L of lead showed a concentration of 192.3 ng/mg. However, when supplied with lead-intoxicated rotifers it was not possible to detect the metal in the prawn.
TABLE III LEAD BIOCONCENTRATIONS IN THE MICROALGAE Nannochloropsis oculata (10 ml PER TRIPLICATE, EVERY 24 H), AND LEAD BIOACCUMULATION IN THE ROTIFER Brachionus plicatilis (n = 300 BY TRIPLICATE, EVERY 24 H), AND PRAWN Macrobrachium rosenbergii (n = 5 PER QUINTUPLICATE).
| Pb (mg/L) | 24 h | 48 h | 72 h | 96 h | 144 h |
| Microalgae (ng/mg) | |||||
| Control | 0.80 ± 0.01 | 0.85 ± 0.01* | 0.86 ± 0.002* | 0.87 ± 0.01* | 0.87 ± 0.01* |
| 0.1 | 5.50 ± 0.41 | 2.49 ± 0.71 | 1.41 ± 0.47 | 1.24 ± 12.82* | 0.63 ± 0.25* |
| 0.5 | 8.53 ± 12.37 | 4.62 ± 2.83* | 2.33 ± 14.24* | 1.81 ± 6.61* | 1.20 ± 0.16* |
| 1 | 15.32 ± 0.18 | 4.57 ± 0.44* | 2.65 ± 0.04* | 1.94 ± 3.81* | 0.99 ± 2.65* |
| 5 | 30.26 ± 78.1 | 17.70 ± 0.89 | 31.65 ± 13.22 | 33.68 ± 40.1 | 31.15 ± 1.72 |
| 10 | 37.70 ± 0.72 | 31.62 ± 5.35 | 32.5 ± 1.55* | 36.84 ± 5.80 | 40.85 ± 5.07 |
| Rotifer fed with algae exposed to lead (µg/mg) | |||||
| 1 | ND | 13.47 ± 18.51 | ND | 12.43 ± 12.00 | 2.62 ± 4.54 (120 h) |
| Prawn fed with algae exposed to lead (ng/mg) | |||||
| 1 | 192.30 ± 5.84 | ||||
ND: not detected.
*Significant (p < 0.05).
Table IV shows that the highest BCF values in the microalgae were observed in the first three concentrations after 24 h and they decreased by increasing the lead concentration; thereafter, they decreased with the exposure time. The rotifers showed the highest BAF (2948) after 48 h when fed with N. oculata exposed to 1 mg/L of lead, while this factor was 42.1 for M. rosenbergii fed with the microalgae exposed to 1 mg/L of lead. In contrast, it was not possible to determine the BAF when fed with the rotifer B. plicatilis.
TABLE IV BIOCONCENTRATION FACTOR (BCF) AND BIOACCUMULATION FACTOR (BAF) OBTAINED FOR THE THREE ORGANISMS EXPOSED TO LEAD USED IN THIS STUDY: MICROALGAE, N = 30 ml, every 24 H; ROTIFER, N = 900, every 24 H; PRAWN, N = 25.
| Pb (mg/L) | 24 h | 48 h | 72 h | 96 h | 120 h | 144 h |
| BCF in algae | ||||||
| 0.1 | 8.68 | 4.06 | 2.13 | 1.86 | - | 0.90 |
| 0.5 | 7.29 | 3.94 | 2.01 | 1.55 | - | 1.08 |
| 1 | 8.58 | 2.59 | 1.55 | 1.23 | - | 0.65 |
| 5 | 5.21 | 3.05 | 5.33 | 5.54 | - | 5.26 |
| 10 | 3.18 | 2.70 | 2.83 | 3.22 | - | 3.56 |
| BAF in rotifers fed with algae lead-exposed | ||||||
| 1 | ND | 2948 | ND | 2720 | 574 | |
| BAF in prawns fed with algae lead-exposed | ||||||
| 1 | 42.1 | |||||
ND: not detected.
DISCUSSION
Lead accumulation in microalgae
This study shows that lead can mobilize through trophic chains. Even though at first lead incorporates into primary producers with the possibility of not affecting their growth, it accumulates and biotransfers in those organisms that feed directly on phytoplankton. This type of study offers an approach to understanding the incorporation, accumulation, and mobilization of metals in food chains, by predicting the fate of the pollutant (Soto-Jiménez et al. 2011). Our results confirm that N. oculata accumulates lead without significantly inhibiting its growth rate during the first 48 h (Fig. 1, Table I), accumulating the highest lead concentration in 24 h with values from 5.5 to 37.7 ng/mg (Table III). Some algae are resistant to metals because they can produce extracellular polysaccharides and use class III metallothioneins as part of their detoxification mechanisms (Perales-Vela et al. 2006). The tolerance of N. oculata to lead is probably a result of these mechanisms. The accumulation of potentially toxic elements such as metals by microalgae involves several mechanisms: (a) adsorption and rapid removal by electrostatic interactions between functional groups of the cell wall, metal ions and specificity joints, and (b) intracellular mechanisms such as covalent bonds, surface precipitation, redox reactions, crystallization of the cellular surface or cellular transport mechanisms (Monteiro et al. 2012, Kumar et al. 2015). The intake of lead by the microalgae occurs through calcium and magnesium transport routes; however, the lead rate intake may be higher if they also use the copper transport route, increasing the sensitivity to lead, e.g., as Chlamydomonas reinhardtii does (Sánchez-Marín et al. 2014). Chlorella vulgaris accumulates lead with a removal efficiency of 98.7 % when exposed to concentrations of 1.95-4.83 mg/L for 1 h; however, at 24, 48, and 72 h of exposure it shows EC50 values of 0.16, 0.49, and 1.85 mg/L (Regaldo et al. 2013). T. suecica is capable of accumulating 18.4 ± 3.4 μg/g of lead in 72 h decreasing its growth by 40 % (Soto-Jiménez et al. 2011). A significant adverse effect was detected when N. oculata was exposed to 1 mg/L for 72 h (p < 0.05) (Fig. 1, Table I). An EC50 value of 5.42 mg/L at 24 h was recorded. The EC50 value decreased with exposure time, suggesting that the microalgae’s growth is significantly inhibited from this concentration onwards (Table II). Also, a value of EC50 (0.23 mg/L) was reported at 96 h in Bold’s medium (freshwater) for N. oculata (Santos-Medrano and Rico-Martínez 2015). We obtained an EC50 value of 3.67 mg/L in the same period in Guillard f/2 medium (15 ‰). The difference may be due to lead bioavailability related to the salinity of the culture medium or maybe to the difference of strains. N. oculata used in this study is less sensitive than other microalgae exposed to lead; however, it begins to accumulates lead from a concentration of 0.1 mg/L, increasing the accumulation rate as the lead concentration increases (Fig. 1, Table III). Also, the highest BCF values in the microalgae were recorded at 24 h, at which point the highest values were obtained at the lowest concentrations (≤ 1 mg/L). Meanwhile, these values decrease by increasing the exposure concentration (Table IV). For this reason, we decided to use concentrations of 0.1 and 1.0 mg/L to accumulate lead, providing a microalga suitable for the consumer and ensuring the transfer of the metal to the following trophic levels. Microalgae of the genus Nannochloropsis are considered a safe dietary supplement with high levels of proteins, pigments, and PUFAs; also, they are used in mariculture and aquaculture as living food (Moha-León et al. 2018, Pérez-Legaspi et al. 2019). The ability of N. oculata to accumulate lead without showing adverse effects can influence the transfer and accumulation of metals in organisms that feed on phytoplankton during their whole life cycles, such as bivalve mollusks. In the Gulf of Mexico, oysters such as C. virginica show maximum lead values of 26.32 µg/g, whereas maximum lead concentrations of 0.158 mg/g are recorded in coastal lagoon sediments (Vázquez-Botello et al. 2004). The Mexican Official Standard NOM-001-ECOL-1996 establishes that the allowable maximum limit of lead in natural and artificial reservoirs for fishing exploitation is 1.0 mg/L per day and 0.2 mg/L per month (SEMARNAT 1997); however, lead bioaccumulation occurs in aquatic organisms used for human consumption that represent a risk to health (Zhang et al. 2016).
Lead accumulation in the rotifer
Lead accumulation in the rotifer B. plicatilis was not detected when it was fed on microalgae exposed to 0.1 mg/L of lead, whereas when microalgae were exposed to 1.0 mg/L of lead, the rotifer accumulated up to 13.47 ± 18.51 µg/mg at 48 h, decreasing over time but accumulating again from 96 h (12.43 ± 12 µg/mg) and decreasing by 120 h (Table III). Rotifers are probably able to excrete the accumulated lead in the microalgae exposed to low concentrations, but accumulate the metal when they consume microalgae exposed to higher lead concentrations for 48 h. After this time, lead is probably excreted by the rotifer. However, chronic feeding on contaminated microalgae can saturate their excretion rate. Therefore, it is advisable to evaluate the excretion rate of potentially toxic elements as metals in rotifers to improve our toxicokinetic knowledge on these organisms. Alvarado-Flores et al. (2012) report that after 24 h of exposure to 1.0 mg/L of lead, the rotifer B. calyciflorus accumulates this metal in the digestive and reproductive systems, forming lead granules. Hernández-Flores et al. (2020) report that rotifers can select their food by avoiding the ingestion of metal-contaminated microalgae, so the toxicity of this food decreases the ingestion rate, which is a very sensitive endpoint in rotifers and may explain the temporal variation in our lead accumulation results. When the rotifer B. plicatilis was fed with N. oculata exposed to concentrations of 0.1 and 1.0 mg/L of lead, its dry weight decreased at 48 h, being significant for 1.0 mg/L (Table I). This decrease in dry weight is probably related to the descaling of the loriga, since the accumulation of this metal replaces calcium with lead obtained from diet (Alvarado-Flores et al. 2012).
Also, reproduction is affected in the rotifer Lecane quadrientata (EC50 = 0.057 mg/L) due to lead accumulation of up to 44 % when consuming N. oculata grown in the Bold’s medium. The microalga grown in freshwater medium probably accumulates more lead than in saline medium, influencing the availability of the metal in the microalga for the rotifer (Hernández-Flores and Rico-Martínez 2006). The estimate of the BAF (2948) in the rotifer B. plicatilis fed with microalgae exposed to 1.0 mg/L of lead was recorded at 48 h, but the metal was not detected the next day. However, in the next 48 h, the BAF reached a value of 2720, decreasing over time to 574 (Table IV). A BAF of 123 684f or Asplanchna was reported for lead (Rubio-Franchini et al. 2011), which may be associated with the trophic level of this rotifer and the place of collection.
Lead accumulation in the prawn
The concentration of lead through diet in the prawn M. rosenbergii is only detectable when fed with N. oculata exposed to 1.0 mg/L of lead, suggesting that the prawn larva may excrete lower concentrations of the metal. Also, when feeding rotifers that consumed lead-exposed microalgae, lead is not detected in the prawn probably because the second trophic level forms unavailable lead granules before reaching the next level (Table III) or because the prawn can remove lead by using deposits of metal granules and excrete it through urine (Vogt and Quinitio 1994). Even the rate of excretion may be higher depending on the efficiency of assimilation of metals, since it depends on how it is incorporated into bone structures, exoskeleton, or granules in the animal with the possibility of being excreted (Soto-Jiménez 2011); in the case of rotifers, lead may be associated with trophi, loriga, or granules influencing their assimilation. Invertebrates can detoxify from metals through various routes: (a) physiological mechanisms that regulate the rate of uptake-excretion, (b) intracellular sequestration mechanisms with metalloproteins affinity and subsequent elimination by the endomembrane system, and (c) intracellular sequestration of metals by specific vacuoles that produce sulfur granules for exocytosis and elimination (Ahearn et al. 2004). In crustaceans, a site of detoxification and sequestration of metals acquired through diet occurs in the epithelial cells of the hepatopancreas (Sterling et al. 2007), where cytoplastic metals are subject to sequestration and detoxification by mitochondria, lysosomes, and endoplasmic reticulum (Ahearn et al. 2004, Sterling et al. 2007). The only BAF value obtained for the M. rosenbergii prawn (42.1) corresponds to the direct ingestion of microalgae exposed to 1.0 mg/L of lead. When the larva was fed with rotifers that consumed the lead-exposed microalgae it was not possible to estimate the BAF (Table IV), even though the rotifer accumulated lead (Table III). The degree of trophic availability of the metals is determined by the efficiency of assimilation of the intestine, the amount ingested, and the chemical form of the metal in the food source (Sánchez-Marín and Beiras 2017). Also, the intervention of sequestration and detoxification mechanisms affects the accumulation of lead in the third trophic level (Ahearn et al. 2004, Sterling et al. 2007). When the formation of lead-rich granules composed by Pb3 (PO4)2 replace calcium, it makes lead unavailable for trophic transfer (Sánchez-Marín and Beiras 2017) and rotifers may form this kind of unavailable lead-rich granules (Alvarado-Flores et al. 2012). This matches the observations of Sánchez-Marín and Beiras (2017), who found that lead-rich granules accumulated in the clam D. exoleta are not available for transfer into the prawn Palaemon serratus. However, the composition of these granules in mollusks, as well as the speciation of lead influence its bioavailability, transfer, and accumulation (Sánchez-Marín et al. 2019). Also, the supplied diet may not favor metal accumulation in crustaceans because it may accumulate metals mainly through gills across epithelial surfaces, since they are an important organ in breathing and osmoregulation (Soegianto et al. 2016). Exposure concentrations of lead at 48 h (16-56 mg/L) in the M. rosenbergii postlarvae show 5.63 g/g in tissue with a BCF of 340.2 (Camacho-Sánchez 2007). Even lead exposure (2-4 mg/L) provokes necrosis and hyperplasia in the M. rosenbergii branchial tissue, reaching LC50 values of 0.47, 0.58, and 2.03 mg/L in postlarvae (PL11), juveniles, and adults, respectively, at 12 ‰ (Soegianto et al. 2016). The transfer and accumulation of trace metals depends on the detoxification mechanisms of each species, as well as their biochemical composition (Soto-Jiménez 2011). Therefore, strict regulatory measures should be adopted to reduce the risk of consuming contaminated aquatic products which affect human health, as well as to diminish environmental hazards due to the recalcitrance, persistence, toxicity, and bioaccumulation of potentially toxic elements (such as metals) in aquatic ecosystems.
CONCLUSION
Lead can accumulate in the microalga N. oculata at low concentrations without inhibiting its growth, which enables it to remain available for the following consumers. This metal can be transferred and concentrated through the direct ingestion of primary producers into the rotifer and prawn, but not when feeding on the primary consumer such as the rotifer, suggesting these invertebrates can immobilize or excrete lead at low concentrations, consequently decreasing its bioavailability.











 nueva página del texto (beta)
nueva página del texto (beta)


