Introduction
The opilionological fauna of Venezuela is the second most diverse in the world, currently with 392 valid species (362 Laniatores, 29 Eupnoi, 1 Dyspnoi), only behind Brazil (1,010 spp.) (A.B. Kury, HarvEx, unpublished data). However, this number of species still seems to be underestimated due to the great undescribed biodiversity of the country, the well-preserved but poorly studied forests areas (e.g., in Amazonia and the Andes), as well as the concentration of sampling (80%-85% of described species) in few localities near major cities (González-Sponga, 1987, 1992), as seen for Laniatores, with Miranda (75 spp.), Distrito Capital (51 spp.), Mérida (49 spp.), and Aragua (48 spp.) as the most collected regions (Kury, 2003). A similar phenomenon occurs in other groups of arachnids, as evidenced by prospecting with interesting results in spiders (Huber & Villarreal, 2020).
Most of this harvestmen diversity in Venezuela has been described by Manuel Ángel González-Sponga (1929-2009), who made a great sampling effort in this country, resulting in 254 valid species to date. Additionally, the other authors who contributed most to the knowledge of the Venezuelan opilionofauna are Roewer (49 spp.), Sørensen (18 spp.), and Caporiacco (16 spp.). Most of this biodiversity, however, is only superficially known, and due to the lack of modern revisionary work, we are still confronted with many taxonomic problems in Venezuelan harvestmen. These should be gradually tackled, especially regarding poorly characterized or unrecognized species.
In order to help improve the taxonomic knowledge of Venezuelan Gonyleptoidea, types of some species described by González-Sponga belonging to the genera Avima Roewer, 1949, OcoitaGonzález-Sponga, 1987 (Agoristenidae), and Phareicranaus Roewer, 1913 (Cranaidae) were studied, and 5 new specific synonymies were detected. Additionally, literature revision and photographs of members of Paecilaema C.L. Koch, 1839 (Cosmetidae), Medellinia Mello-Leitão, 1939, Phareicranaus Roewer, 1913, StygnicranellaCaporiacco 1951 (Cranaidae) and Rhopalocranaus Roewer, 1913 (Manaosbiidae), led to the discovery of 1 generic and 4 specific synonyms. In this paper, we propose the nomenclatural changes needed to rectify the status of the aforementioned taxa. Finally, some considerations about distributional patterns noted in Venezuelan harvestmen are made.
Materials and methods
Individuals of the species were imaged from varied sources, as the present extremely unfavorable context makes standardization difficult to attain (e.g., the devastating fire in the National Museum of Rio de Janeiro; many government policies contrary to environmental conservation; the pandemic). We mostly used Nikon 5200, Canon PowerShot S3IS, and Sony Cybershot DSC-V1 cameras attached to a stereomicroscope. The multiple images of each species at different focal planes were combined with Zerene Stacker or CombineZP to increase the depth of field and were then edited in Adobe Photoshop CC2014 software. Scanning Electron Microscopy (SEM) was carried out with a JEOL JSM-6390LV microscope at the Center for SEM of the Museu Nacional/UFRJ with an accelerating voltage of 10 kV after sputter-coating with gold-palladium. All measurements are in mm unless otherwise noted.
For the shape of the dorsal shield we used the classification proposed by Kury and Medrano (2016) and for the morphology of the penis we followed Kury and Villarreal (2015). When listing the examined material, countries are written in capital letters and the first order administrative divisions are written in italics. Geographic coordinates have been transcribed verbatim from the labels and may be in different formats. When no original indications of coordinates were available, we estimated those using GoogleMaps and placed them in decimal degrees between square brackets. The distribution map was made using ESRI ArcGIS 10.4. Colored shapes refer to WWF Terrestrial Ecoregions of the World (Olson et al., 2001).
Abbreviations are: DS = dorsal scutum, G-S = González-Sponga, MS = macrosetae of penis. Cited repositories are: AMNH = American Museum of Natural History, New York, USA; MAGS = former Manuel Angel González Sponga private collection (donated to MIZA), MIZA = Museo del Instituto de Zoología Agrícola “Francisco Fernández Yépez”, Maracay, Venezuela; MBUCV = Museo de Biología de la Universidad Central de Venezuela, Caracas, Venezuela; MCNC = Museo de Ciencias Naturales de Caracas, Caracas, Venezuela; MHNG = Muséum d›histoire naturelle de la Ville de Genève, Geneva, Switzerland; MNRJ = Museu Nacional, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil; MZTU = Museo di Zoologia, Instituto di Zoologia e Anatomia Comparata Universitá di Torino (also MZUT, ZMT, now deposited in the Museo Regionale di Scienze Naturali di Torino (MRSN), which belongs to the Regione Piemonte and not to the University), Torino, Italy; ZMB = Museum für Naturkunde der Humboldt-Universität zu Berlin, Berlin, Germany. The material lost in the National Museum fire (2-September-2018) is marked with an asterisk (*).
Descriptions
Family Agoristenidae Šilhavý, 1973
Subfamily Leiosteninae Šilhavý, 1973
Genus Avima Roewer, 1949
A complete synonymic list may be found in Villarreal and Kury (2009).
Type species: Avima leucobunus Roewer, 1949 from Suriname, Paramaribo.
Avima quadrata (González-Sponga, 1987) (Figs. 1, 2, 17A)
Vima quadrataGonzález-Sponga, 1987: 529, figs. 684-689.
Trinella quadrata - Pinto-da-Rocha, 1996: 319.
Trinella quadrata - Kury, 2003: 33.
Avima quadrata: Villarreal and Kury, 2009: 67.
Vima nigromaculataGonzález-Sponga, 1998: 28, figs. 19-24. Syn. nov.
Trinella nigromaculata-Kury, 2003: 33. Avima nigromaculata - Villarreal and Kury, 2009: 66.

Figure 1 Habitus of Avima quadrata, male (MNRJ 9453): A, panoramic view; B, dorsal view; C, lateral view; D, anterior view; E, ventral view; F, detail of hooked anterior tubercle of coxa I. Scale bars: A-C, E, 1 mm, D, 0.5 mm, F, 0.2 mm.
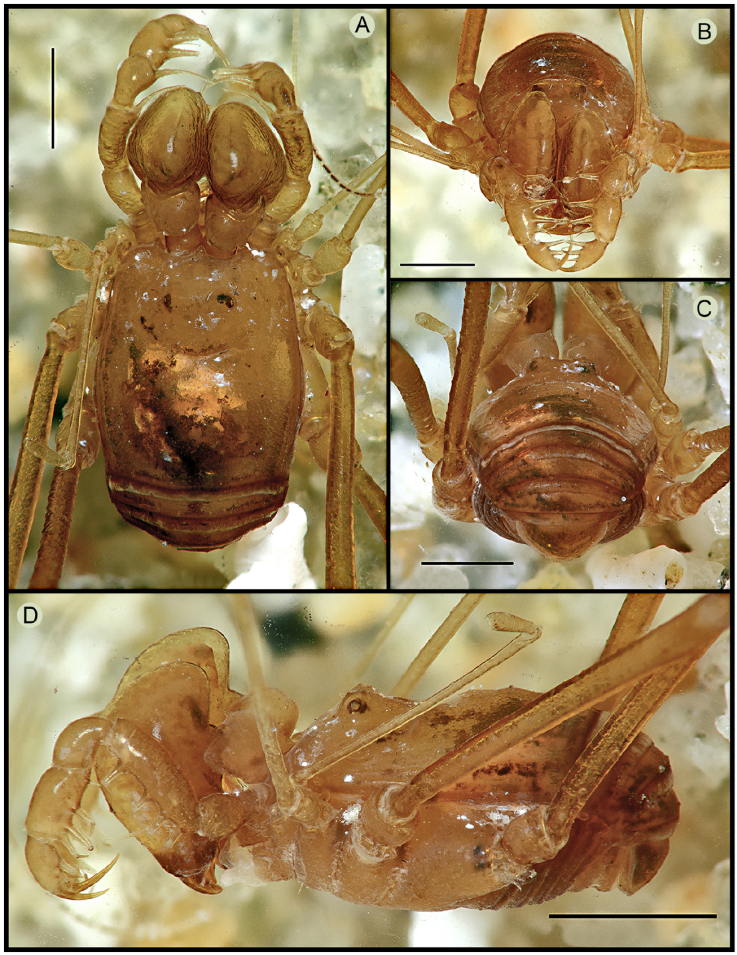
Figure 2 Habitus of Avima nigromaculata, male holotype (MAGS 1161a): A, dorsal view; B, anterior view; C, posterior view; D, lateral view. Scale bars: 1 mm.
Taxonomic summary
Type data: Avima quadrata: Venezuela, Lara, Morán, environs of Guarico [9.621126° -69.787648°], 1,100 m, 25.x.1980, Nerys Quiroz and Tito Quiroz leg., 1♂ holotype (MCNC 977); same data as holotype, 1♀ paratype (MCNC 978); same locality as holotype, 14.ii.1983, A.R. Delgado de González, J.A. González Delgado and M.A. González-Sponga leg., 6♂ 6♀ 5 juv. paratypes (MAGS). Avima nigromaculata: Venezuela, Trujillo, Boconó, Mosquei [Mosquey], near Boconó, 9°14’40.0” N, 70°15’46.0” W [9.244444° -70.262778°], 2,450 m, 18.ii.1991, A.R. Delgado de González, Emigdio González and M.A. González-Sponga leg., 1♂ holotype (MAGS 1161a); same data as holotype, 1♀ paratype (MAGS 1161b); 5♂ 5♀ paratypes (MAGS).
Records: Venezuela, Lara, Jiménez, near Cubiro and Parque Nacional Yacambú [9.783582° -69.584401°; 1,500 m] (González-Sponga, 1987).
Material studied: Venezuela, Lara, Parque Nacional Yacambú [9.633333° -69.666667°; 1,600 m], xii.2002, A. Pérez and A. Giupponi leg., 13♂ 20♀ (MNRJ 9453*); Trujillo, near Boconó, Laguna Negra (9.3054° -70.1752°), 1,870 m asl, 21.xi.2018, O. Villarreal M. and B.A. Huber leg., 1♂.
Distribution: in the rainforests of Cordillera de Mérida, in Venezuelan Andes montane forests ecoregion (NT0175) (Fig. 17).
Remarks
The penises of the individuals here studied were dissected by G-S. Unfortunately, no vials with genitals were found among the specimens. Possibly, the genitalia of this species are deposited in individualized vials in other container glasses, a method that G-S used to preserve the Zalmoxidae/Samoidae material from his collection.
González-Sponga (1998: 30) says “Vima nigromaculata differs from Vima quadrata González-Sponga, 1987, the closest geographic species, because of: a) shape of dorsal scutum; b) armature of ocularium and c) general shape of male genitalia”. However, we found that the morphology of both species is similar, mainly based on: a) the DS Epsilon type and the position of the dark blot is identical in both species (Figs. 1B, D, 2A, B); b) the ocularium has some dispersed granules in both species, not having any relevant ornamentation (Figs. 1C, 2D); c) the size of the femora is the same for both male holotypes (4.50 mm according to G-S); d) even when the drawings of the male genitalia of A. nigromaculata are quite minimalist (González-Sponga, 1998: figs. 21-22), it is possible to see the similarity of the dorsal keel of the stylus and one MS-A close to the base of the stylus, present in the penis of A. quadrata too. Besides that, the morphological similarities are in accordance with the type localities, which are separated by no more than 50 km and are immersed in the same biogeographic region, exhibiting an ecological continuum.
Genus OcoitaGonzález-Sponga, 1987
OcoitaGonzález-Sponga, 1987: 456; Kury, 1997b: 344; Kury, 2003: 31.
Type species: Ocoita minaGonzález-Sponga, 1987, by original designation.
Ocoita tapipensisGonzález-Sponga, 1987 (Figs. 3, 4, 17A)
Ocoita tapipensisGonzález-Sponga, 1987: 461, figs. 586-592.
Ocoita tapipensis - Kury, 2003: 31.
Ocoita servaeGonzález-Sponga, 1987: 465, figs. 593-599. Syn. nov.
Ocoita servae - Kury, 2003: 31.

Figure 3 Habitus of Ocoita tapipensisGonzález-Sponga, 1987, male (MAGS 728): A, panoramic view; B, dorsal view; C, lateral view. Scale bars: 1 mm.
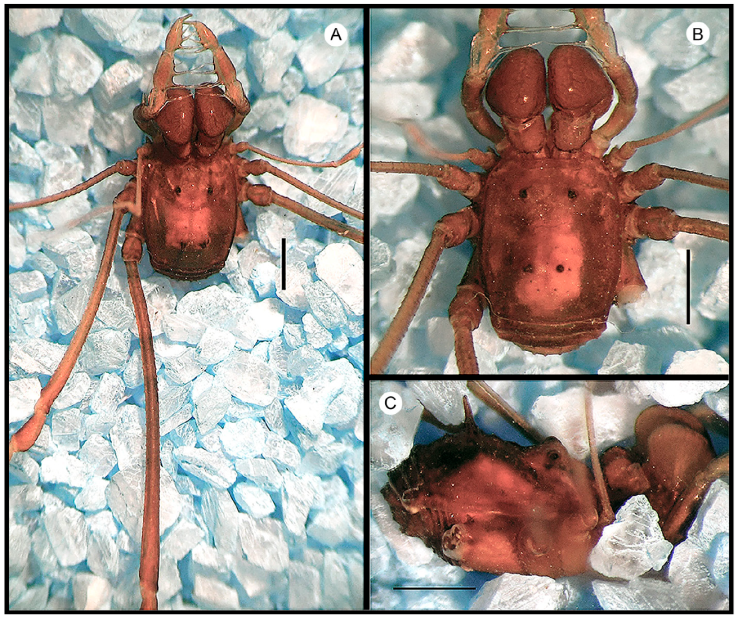
Figure 4 Habitus of Ocoita servaeGonzález-Sponga, 1987, male (MAGS 729): A, panoramic view; B, dorsal view; C, lateral view. Scale bars: 1 mm.
Taxonomic summary
Type data: Ocoita tapipensis: Venezuela, Miranda, Acevedo: Boca de Curia [misspelling as Boca de Cuira by González-Sponga], 10.203780° -66.291889°, 50 m, A.R. Delgado de González, J.A. González Delgado and M.A. González-Sponga leg., 1♂ holotype (MCNC 959), not examined; 1♀ paratype (MCNC 960), 5♂ 5♀ paratypes (MAGS), same data as holotype. Ocoita servae: Venezuela, Miranda, Paéz/Acevedo: environs of Cumbo [10.229826° -66.084180°], 50 m, A.R. Delgado de González, J.A. González Delgado and M.A. González-Sponga leg., 1♂ holotype (MCNC 961), not examined; 1♀ paratype (MCNC 962); 1♂ 6♀ paratypes (MAGS), same data as holotype.
Distribution: in Cordillera de la Costa montane forest (NT0117) and La Costa xeric shrub lands (NT1309) ecoregions (Fig. 17).
Remarks
Male exemplars MAGS 728 and MAGS 729 deposited in MIZA collection were examined and used for the complementary description (Figs. 3, 4). We suspect that these males correspond to the male paratypes cited above as MAGS, without number. The individuals studied here had been dissected previously; however, no vials with the genital were found together. Possibly, the genitalia of this species are deposited in individualized vials, in other container glasses, a method that G-S used to preserve the Zalmoxidae/Samoidae material from their collection, however, they were not found.
G-S differentiated O. tapipensis from O. servae based mainly in the presence of a granule in the anteroproximal tubercle of coxa I and the granulation of mesotergal areas in the latter. However, we found the same kind of granulation (2 paramedian granules) on areas I, II, and IV, and a simple tubercle on coxa I (without conspicuous granule) in both species.
Family Cosmetidae C.L. Koch, 1839
Genus Paecilaema C.L. Koch, 1839
A complete and updated synonymic list is found in Kury and Medrano (2018).
Type species:Cosmetus u-flavumPerty, 1833, by subsequent designation of Pickard-Cambridge (1905: 570). “Paecilaema” sexlineatumGoodnight and Goodnight, 1942 (Figs. 5, 6, 17A)
Paecilaema sexlineataGoodnight and Goodnight 1942: 5, fig. 9.
Paecilaema sexlineatum: Kury 2003: 79 (correction of adjectival inflection to match the neuter gender of genus name).
Paecilaema vittataGonzález-Sponga 1992: 397, figs. 523-528 (junior secondary homonym of Rhaucus vittatus Sørensen, 1932). Syn. Nov.
Paecilaema vitatta: González-Sponga, 1992: 398 (original incorrect spelling).
Paecilaema gonzaleziKury 2003: 76 (nomen substitutum for Paecilaema vittataGonzález-Sponga, 1992). Syn. nov.
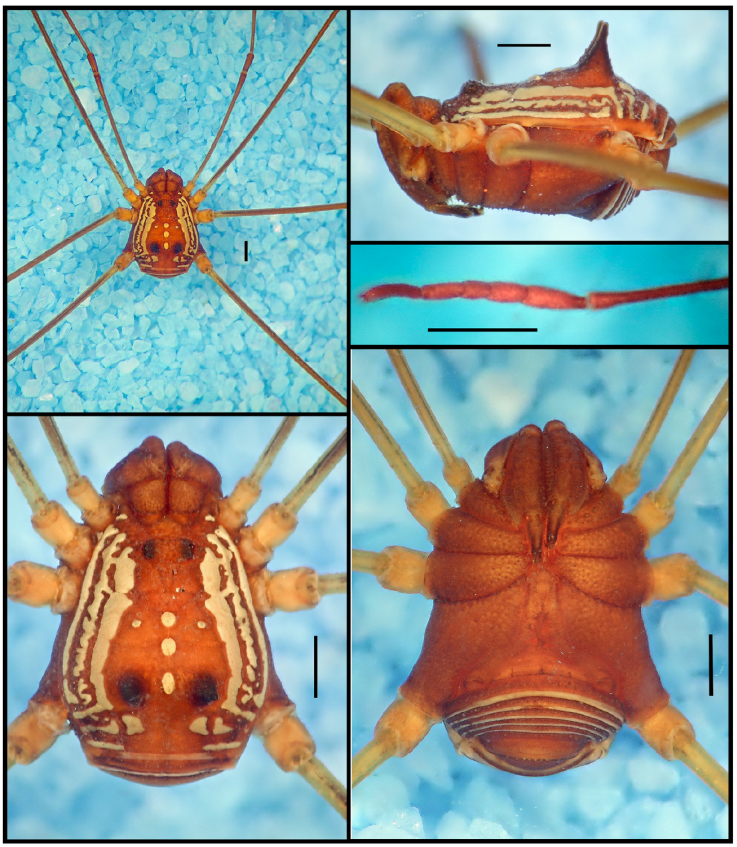
Figure 5 Habitus of Paecilaema sexlineatumGoodnight and Goodnight, 1942, male paratype (AMNH): A, panoramic view; B, dorsal view; C, lateral view; D, ventral view; E, detail of tarsomeres of leg I. Scale bars: 1 mm.

Figure 6 Habitus of living specimens of Paecilaema sexlineatumGoodnight and Goodnight, 1942 from Guyana: A, male, dorsal view; B, same, lateral view; C, same, dorsolateral, posterolateral view; D, female, dorsal view; E, juvenile, dorsolateral view; F, male, anterolateral view. Photographs courtesy of Sèbastien Cally.
Taxonomic summary
Type data: Paecilaema sexlineata: Guyana, Kaieteur [5.202136° -59.475897°], ♀ holotype (AMNH). Paecilaema gonzalezi: Venezuela, Bolívar, Roscio: km 85 road El Dorado-Santa Elena de Uairén [6.186116° -61.411348°], type MAGS 716a, ♂ holotype.
Records: Guyana, Tukeit (Goodnight & Goodnight, 1942).
Distribution: in Guayanan Highlands moist forests (NT0124) ecoregion (Fig. 17), Guyana and Venezuela.
Remarks
Details of the descriptions of both species agree in minutiae, except for body size and legs length, which differ significantly. See for example the shape, the ornamentation, and the pattern of yellow spots of the dorsal scutum (Figs. 5A-C, 6A-D). We still decided to carry on with the proposed synonymy, knowing that in Laniatores there may be wide gaps in dimensions for populations far apart.
Recently, Kury and Medrano (2018) studied some species of the genus Paecilaema, in order to designate a neotype to anchor its type species. They highlighted the difficulty represented by the study of the entire group and described the chaotic taxonomic panorama in the genus Paecilaema and the family in general. With the exception of a few groups (e.g., Neocynorta Roewer, 1915, TaitoKury and Barros, 2014, Rhaucus Simon, 1879, or Roquettea Mello-Leitão, 1931) most genera in the family are meaningless, being made up of artificial groupings. Paecilaema sexlineatum does not fit perfectly with the current diagnosis of the genus, as defined in Kury and Medrano (2018), and rather, some of its characteristics, such as the shape of the dorsal scutum or the long and thin legs, slightly resemble some genera such as Flirtea C.L. Koch, 1839 or Cynorta Koch, 1839. Due to the current taxonomic situation and the absence of a detailed description of the male genitalia of this species, we prefer not to propose any generic changes, but its maintenance in Paecilaema must be provisional.
The few cosmetid genera studied in detail in a modern taxonomic context, until now, seems to show a short range distribution, associated to a greater or lesser degree with well defined biomes. This is particularly obvious in areas with marked environmental heterogeneity, such as the Andes (Neocynorta Roewer, 1915, in the Venezuelan Andean mountain forests; Rhaucus Simon, 1879, in Colombian northern páramo and Cordillera Oriental montane forests) (García & Kury, 2017; Medrano et al., 2019), and less noticeable in regions with more subtle environmental heterogeneity such as the Amazon region. (e.g., TaitoKury and Barros, 2014 and Ampycus Simon, 1879 in the Amazon forests of Brazil, Colombia, and Ecuador) (García, 2014; Kury & Barros, 2014). The distribution of these and other morphologically similar species in the Guiana Shield (i.e., Paecilaema triangulatumGoodnight & Goodnight, 1942; P. lutziGoodnight & Goodnight, 1942; and P. reticulatumGoodnight & Goodnight, 1942) seems to indicate the existence of a group of related species, which may not be part of Paecilaema and need their own generic definition, or a redefinition of current diagnoses of pre-existing groups, for example Flirtea C.L. Koch 1839. Lastly, it is interesting to note that both populations of “Paecilaema” sexlineatum treated here are not so far apart (about 200 km in a straight line) and in fact, they are on the border between neighboring ecoregions, which could explain this apparent wide distribution.
Family Cranaidae
Genus Phareicranaus Roewer, 1913
A complete synonymic list is found in Pinto-da-Rocha and Bonaldo (2011).
Type species: Goniosoma calcariferum Simon, 1879, by original designation.
Phareicranaus albilineatus (Roewer, 1916) (Figs. 7-10, 17)
Santinezia albilineataRoewer, 1932: 290, fig. 7 [junior subjective synonym of Inezia curvipesRoewer, 1916 by Pinto-da-Rocha & Kury (2003: 198); synonymy rejected by Villarreal & Rodriguez (2011: 2204)].
Santinezia albilineata - Soares & Soares, 1948b: 617; Goodnight & Goodnight, 1949: 23;
Caporiacco, 1951b: 27; Rambla, 1978a: 8; Avram, 1987: 87; Decu et al., 1987: 34; Rambla & Juberthie, 1994: 221; González-Sponga, 2003: 41; Villarreal & Rodriguez, 2011: 2204, images 6-10; figs 7-9.
Phareicranaus albilineatus: Pinto-da-Rocha & Bonaldo, 2011: 6.
Santinezia francourbaniiAvram, 1987: 83, figs 5-11 [junior subjective synonym of Inezia curvipesRoewer, 1916 by González-Sponga (2003: 42); synonymy reaffirmed by Pinto-da-Rocha & Kury (2003: 198)]. Syn. nov.
Santinezia francourbanii - Rambla & Juberthie, 1994: 221.
Santinezia francourbani: González-Sponga, 2003: 2, 42; Pinto-da-Rocha & Kury, 2003: 198. [Incorrect subsequent spelling].
Santinezia decuiAvram, 1987: 86, figs. 16-19 [junior subjective synonym of Inezia curvipesRoewer, 1916 by Pinto-da-Rocha & Kury (2003: 198); junior subjective synonym of Santinezia albilineata Roewer, 1932 by González-Sponga (2003), synonymy reaffirmed by Villarreal & Rodriguez (2011: 2204)].
Santinezia guaricoensisGonzález-Sponga, 2003: 32, figs 70-75. Syn. Nov.
Phareicranaus guaricoensis: Pinto-da-Rocha & Bonaldo, 2011: 17.
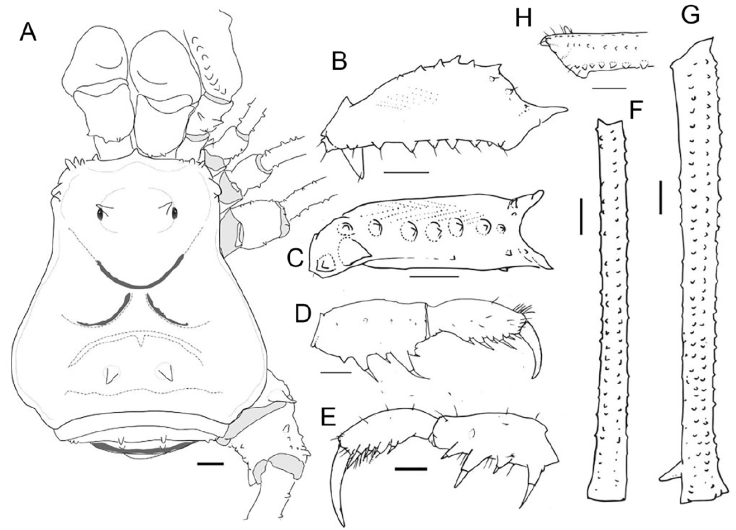
Figure 7 Phareicranaus albilineatus (MNRJ 7916*) from Quebrada Camburales, Miranda: A, habitus, dorsal view; B, right pedipalpal femur, ectal view; C, same, ventral view; D, right pedipalpal tibia, tarsus and claw, dorsomesal view; E, same, ectal view; F, Tibia IV, dorsal view; G, femur IV, dorsal view; H, same, detail of the distal portion, prolateral view. Scale bars: 1 mm.
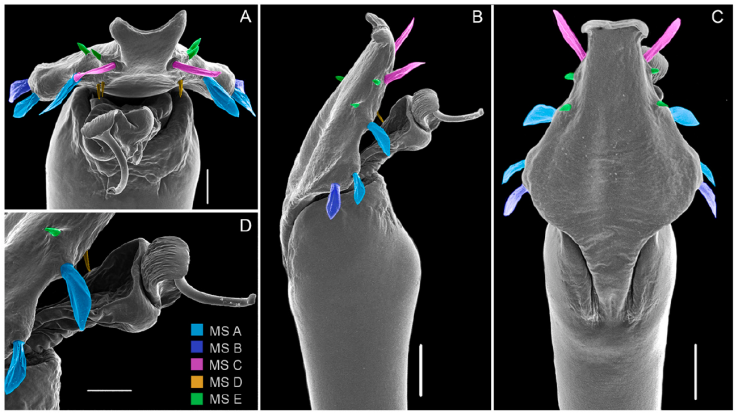
Figure 8 Phareicranaus albilineatus, from Quebrada Camburales, Miranda (C, D): A, dorsal view; B, lateral view; C, ventral view. MS = Macro Setae of the ventral plate. Scale bars: 100 µm.
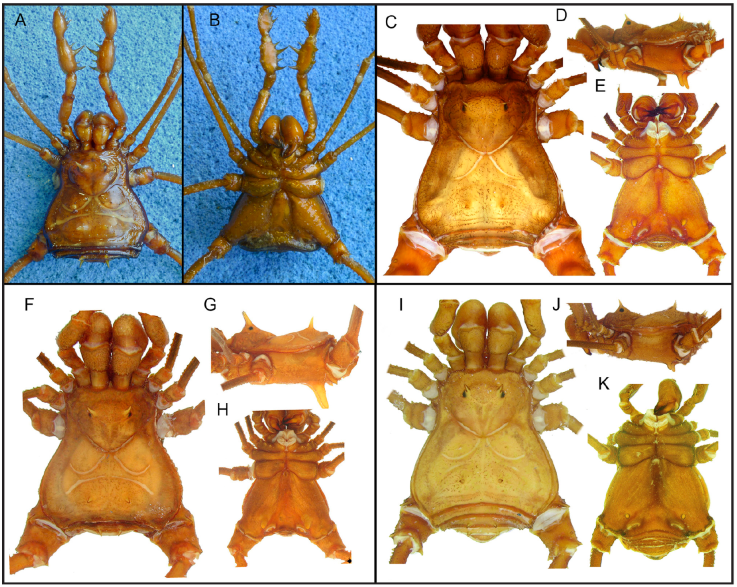
Figure 9 Santinezia albilineata (currently Phareicranaus albilineatus), female holotype (ZMB 7468), habitus: A, dorsal view; B, ventral view; C, D, E, Phareicranaus albilineatus, male from Tiara, Aragua (MNRJ 7116); F, G, H, Phareicranaus capayitaensis, male holotype (MAGS 179a), Miranda, dorsal, lateral and ventral view; I, J, K, Santinezia guaricoensis, male holotype (MAGS 282a), from Guárico, dorsal, lateral and ventral view. Photographs A, B courtesy of Ricardo Pinto-da-Rocha.

Figure 10 Living specimens of Phareicranaus albilineatus (Roewer, 1916) from Bosque de la Virgen, Jardines Topotepuy, Miranda: A, C, females; B, male. Photographs Osvaldo Villarreal.
Taxonomic summary
Type data: Santinezia albilineata: Venezuela, Aragua, San Casimiro [9.999882° -67.011890°], ♀ holotype (ZMB-7468) (examined by photos). Santinezia capayitaensis: Venezuela, Miranda, Zamora [Araira], Hacienda Capayita [10.456920° -66.473678°], ♂ holotype (MAGS-179a) (examined), ♀ paratype (MAGS-179b) and 50♂, 90♀, 128 juv. same data (not examined); Santinezia decui: Venezuela, Aragua, Tiara [10.131518° -67.154960°], ♀ holotype (ISER); Santinezia francourbanii: Venezuela, Miranda, El Hatillo, Cueva de la Esmeralda [10.435372°-66.778821°]. ♂ holotype, 1 juv. paratype, 3♀ immatures, paratypes (not examined). Santinezia guaricoensis: Venezuela, Guárico, Monagas, Morrito Arriba, carretera a San Francisco de Macaira [9.930500° -66.300598°], ♂ holotype (MAGS-282a), (examined); same ♀ (MAGS-282b) (not examined) and same 5♂, 22♀, 3 juv. (not examined).
Other material studied: Venezuela: Miranda, Caracas, Hacienda La Trinidad [10.431054° -66.858997°], 28.xii.1970, W. Peck leg., shallow cave, 1,500 m 3♂ 1♀ (CAS, without number*); Miranda, Quebrada de Cambural [10.400000° -66.916700°], xii.2002, Pérez A., Giupponi A. leg. 15♂ 16♀ 17 juv. (MNRJ 7916*); Miranda, Bosque de La Virgen, Jardines Topotepuy [10.417483° -66.851193°], 11.xi.2019, O. Villarreal M., J. Rodríguez leg. 1♂, 1♀ (MIZA, without number).
Distribution: in the cloud forests (Fig. 18A, B) of Cordillera de la Costa montane forest (NT0117) and La Costa xeric shrub lands (NT1309) ecoregions (Fig. 17).
Remarks
Although Phareicranaus francourbanii was kept as a junior subjective synonym of Phareicranaus curvipes by several authors (Pinto-da-Rocha & Bonaldo, 2011; Pinto-da-Rocha & Kury, 2003; Villarreal & Rodríguez, 2011), we decided here to consider it conspecific with P. albilineatus, mainly due to: penis with MS A1-2 spatulate and aligned with MS B on the edge of the VP (see Avram 1987: figs. 10-11), not MS A1-2 grouped with MS D, and separated from MS B, as is observed in P. curvipes (Fig. 13); the shape of the outline of dorsal scutum and pattern of yellow strips (Figs. 7A, 9A, B, 10). Other characters that allow separating both species can be checked in Villarreal and Rodríguez (2011).
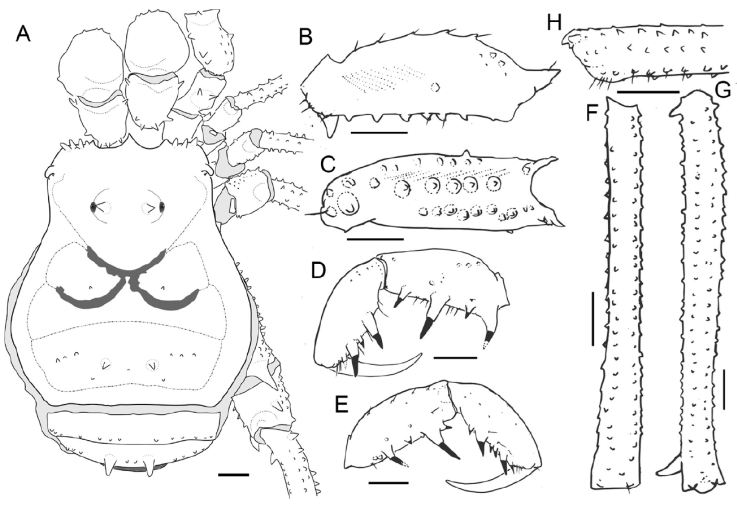
Figure 11 Phareicranaus curvipes (MNRJ 18988*) from Rancho Grande, Aragua: A, habitus, dorsal view; B, right pedipal femur, ectal view; C, same, ventral view; D, right pedipalpal tibia, tarsus and claw, dorsomesal view; E, same, ectal view; F, Tibia IV, dorsal view; G, femur IV, dorsal view; H, same, detail of the distal portion, prolateral view. Scale bars: 1 mm.
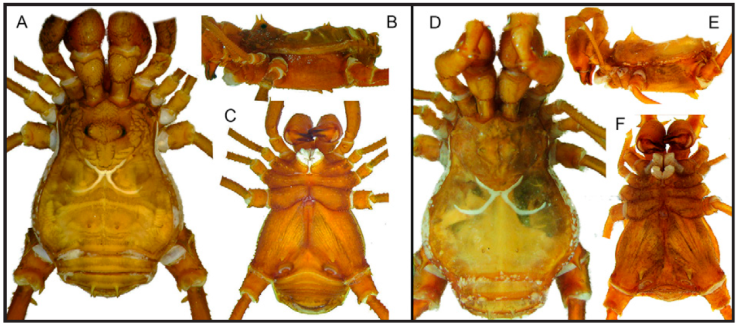
Figure 12 Phareicranaus curvipes, from Rancho Grande, Aragua (A-C) and P. leonensis, holotype MAGS-10a (D-F): A, D, dorsal view; B, E, lateral view; C, F, ventral view.
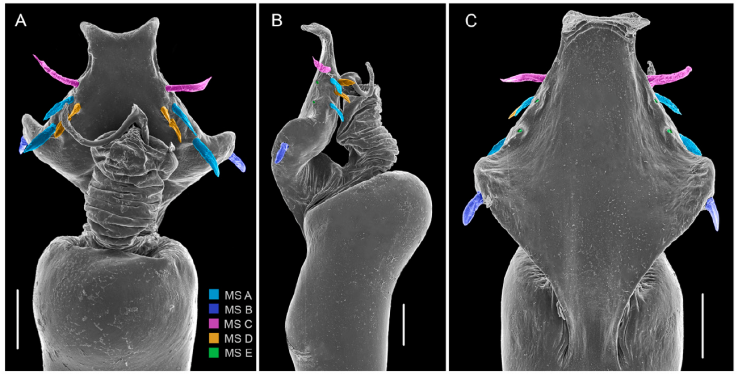
Figure 13 Phareicranaus curvipes, from Rancho Grande, Aragua: A, dorsal view; B, lateral view; C, ventral view. MS = Macro Setae of the ventral plate. Scale bars: 100 µm.
Intraspecific variations in Gonyleptoidea have been recorded in several groups, e.g., the white/yellow dorsal blots of Cosmetidae (Kury & Barros, 2014), the body projections considered secondary sexual characteristics in Cranaidae (Villarreal et al., 2015), or the projections of the scutal areas in Cosmetidae (Medrano & Kury, 2018). In the type specimens here studied we found variations related to size of the spines on ocularium and mesotergal area III, and width/length of the yellow spots on the dorsal scutum (Fig. 9). The specimens from Miranda described by G-S as S. capayitaensis (Fig. 9F-H) exhibit larger spines and a more complete blot on scutal groove II when compared with those from Tiara (Aragua) (Fig. 9C-E) and Guárico (Fig. 9I-K), but not much different from the female holotype from Casimiro (Aragua) (Fig. 9A, B). However, the common sexual dimorphism in the color pattern of Phareicranaus should be taken into account, where females generally have the most complete and wider stripes, which makes comparison difficult between those holotypes. We have not observed notable differences in the morphology of the penis between the populations that we were able to study. All this leads us to propose the synonym of these species under P. albilineatus.
On the other hand, in some Gonyleptoidea species we can find 2 male morphotypes, called major/alfa and minor/ beta, according to the degree of expression of the secondary sexual traits, each with different reproductive strategies (Buzatto & Machado, 2014). Population density can affect agonistic encounter rates between territorials (majors) and also the number of sneakers (minors) invading harems. Therefore, environmental and demographic parameters may influence the relative fitness of each male tactic and ultimately may determine whether the population will be composed of dimorphic or monomorphic males (Tomkins & Brown, 2004).
The proportion of major and minor males varies between populations. For example, in the species Serracutisoma proximum (Mello-Leitão, 1922), the ratio of minor males to the total number of males (taking into account differences in detectability) ranged from 5.8% to 36.7% among populations, with a mean of 16.9% (Munguía-Steyer et al., 2012). In the case of P. capayitaensis, a high population density is inferred from the numerous type series (G-S collected 50 males, 90 females, and 128 subadults), possibly, leading males to exhibit the greatest sexual dimorphism within the specimens studied. The study of isolated populations with highly dimorphic males and minor males and ignorance of these variations could have guided the authors to recognize these populations as distinct species.
Phareicranaus benedictoi (Soares and Avram, 1981) comb. nov.; nomen inquirendum; revalidated.
Santinezia benedictoiSoares and Avram, 1981: 95 (junior subjective synonym of Inezia curvipesRoewer, 1916 by Pinto-da-Rocha and Kury [2003: 198]).
Taxonomic summary
Type data: ♂ holotype (possibly in MZTU), Venezuela, without further locality data.
Remarks
Soares and Avram (1981) presented an extremely short description of this species (which they characterize as a pre-description), containing an abridged diagnosis and lacking any indication of type locality other than “Venezuela”. In spite of the terseness of Soares and Avram text, it qualifies as a description sensu ICZN because it appeared in a publication, it is indicated as a new species, and it has a diagnosis. Later, González-Sponga (2003) wrongly disqualified this species as “inválida” [= invalid], (when he surely meant “unavailable”, although he did not used the term “nomen nudum”), because he argued that it lacked a detailed type-locality and a lengthy description. Pinto-da-Rocha and Kury (2003), based only on the description, synonymized this species with S. curvipes. However, by extracting data from the squalid diagnosis, we found S. benedictoi to be similar to P. albilineatus, although it can be distinguished by: 1) free tergite I with paramedian tubercles, 2) free tergite III unarmed, and 3) armature of legs and pedipalps “different” (without any comment on how it is different). This combination of characters excludes its identity with both P. albilineatus and P. curvipes. However, it matches some species from the Venezuelan Andes.
Phareicranaus curvipes (Roewer, 1916) (Figs. 11-14, 17)
Inezia curvipesRoewer 1916: 8.
Santinezia curvipes: Roewer, 1923: 553; Roewer, 1932: 553.
Phareicranaus curvipes: Pinto-da-Rocha and Bonaldo, 2011: 12.
Goniosoma pavaniMuñoz-Cuevas, 1972: 28, figs. 1-13; Muñoz-Cuevas, 1973: 232, fig. 7.
Santinezia francourbaniAvram, 1987: 83, figs. 5-11; Rambla and Juberthie, 1994: 221.
Santinezia orghidaniAvram, 1987: 85, figs. 12-15.
Cranaostygnus marcuzziCaporiacco, 1951: 26, fig. 14; Kury, 1995: 31.
Santinezia leonensisGonzález-Sponga, 2003: 39, figs. 82-88. Syn. nov.
Phareicranaus leonensis: Pinto-da-Rocha and Bonaldo, 2011: 19.
Santinezia francourbaniAvram, 1987: 83, figs. 5-11 (junior subjective synonym of Inezia curvipesRoewer, 1916 by González-Sponga (2003); synonymy reaffirmed by Pinto-da-Rocha and Kury [2003]).
Santinezia francourbani - Rambla and Juberthie 1994: 221.
Santinezia orghidaniAvram, 1987: 85, figs. 12-15 (junior subjective synonym of Cranaostygnus marcuzziCaporiacco, 1951 by González-Sponga (2003); junior subjective synonym of Inezia curvipesRoewer, 1916 by Pinto-da-Rocha and Kury (2003)).
Cranaostygnus marcuzziCaporiacco, 1951: 26, fig. 14 [junior subjective synonym of Santinezia curvipes by Pinto-da-Rocha and Kury (2003) ].
Cranaostygnus marcuzziKury, 1995: 31. Santinezia leonensisGonzález-Sponga, 2003: 39, figs. 82-88. Syn. nov.
Phareicranaus leonensis - Pinto-da-Rocha and Bonaldo, 2011: 19.

Figure 14 Living specimens of Phareicranaus curvipes (Roewer, 1916) from P.N.H.P., Aragua: A, male; B, female. Photographs Osvaldo Villarreal.
Taxonomic summary
Type data: Cranaostygnus marcuzzii: ♂ juv. holotype (MBUCV 499) Venezuela, Aragua, Rancho Grande, (lost, Rubén Candia pers. comm. 2009). Santinezia leonensis: Venezuela, border line between Distrito Capital and Miranda [La Guaira state sensu Geonames], Alto de Ño León [10.42946° -67.16608°], road El Junquito - Colonia Tovar, ♂ holotype (MAGS-10a) (examined).
Material studied: Venezuela: Aragua, Estación Biológica de Rancho Grande, Parque Nacional Henri Pittier [10.349802° -67.684276°], 12.v.2007, P. Colmenares leg., 1 ♂ (MNRJ 18988*); Henri Pittier National Park near Rancho Grande, 1,100 - 1,800 m, 12 - 30.xi.1997 T. Pape leg. Ex. Museum Estocolmo, 2 ♂ 1 ♀ (MNRJ 5606*).
Distribution: cloud forests (Fig. 18C, D) of Cordillera de la Costa montane forest (NT0117) and La Costa xeric shrublands (NT0124) ecoregions (Fig. 17).
Unlikely record: Falcón, Petit: Uria, Cueva El Coy-Coy (Rambla, 1978). Very likely, this is a misidentification of Phareicranaus heliae Avram, 1983, a common species from that locality.
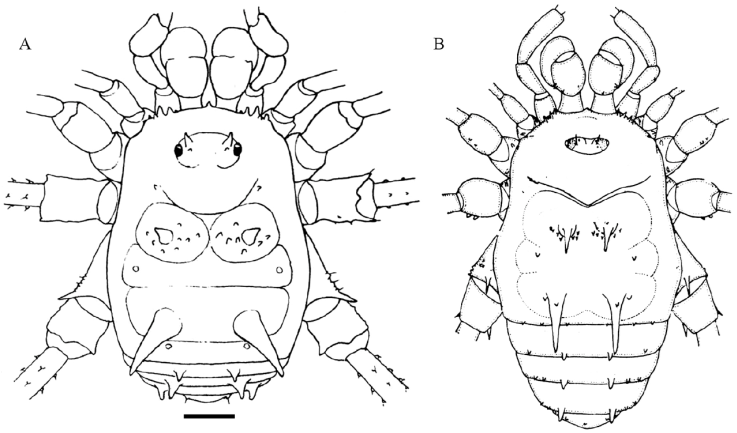
Figure 15 Illustrations from the original descriptions, habitus, dorsal view: A, Rhopalocranaus bordoniŠilhavý, 1979; B, Medellinia bordoniSoares and Avram, 1987. Scale bar: 1 mm. Drawings not to scale.
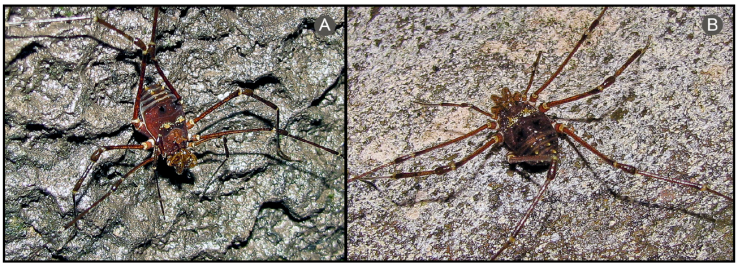
Figure 16 Living specimens of Rhopalocranaus flaviaculeatus (Caporiacco, 1951), females: A, from Colonia Tovar, Aragua; B, from P.N. Henri Pittier, Aragua. Photographs Osvaldo Villarreal.
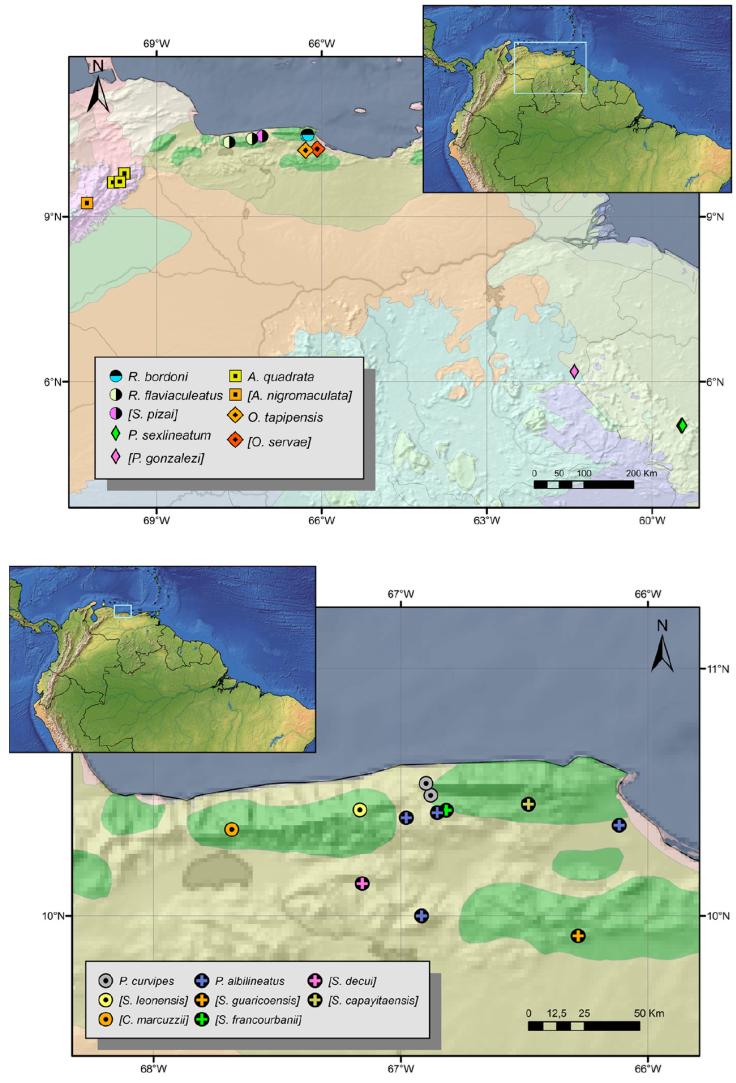
Figure 17 Distribution of relevant species in Venezuela and Guyana. A, Avima quadrata, Ocoita tapipensis, Paecilaema sexlineatum and Rhopalocranaus bordoni. B, Phareicranaus albilineatus and P. curvipes. Each species share the same symbols, but the points representing the holotypes of synonymized (invalid) species have different colors. In the legend, species names in square brackets indicate synonymized (invalid) species aiming to mark their type-localities. The senior synonyms are not enclosed in brackets. Shades in the background represent WWF ecoregions.
Remarks
The holotype of P. leonensis studied here fits into the diagnosis of P. curvipes as defined by Villarreal and Rodríguez (2011), both the external morphology and the male genitalia.
Family Manaosbiidae
Genus Rhopalocranaus Roewer, 1913
A complete synonymic list may be found in Kury (2003).
StygnicranellaCaporiacco, 1951: 24; Soares and Soares 1985: 195; Kury 1995: 31 (type species: Stygnicranella pizaiCaporiacco, 1951, by monotypy). Syn. nov.; attr. nov.
Remarks
StygnicranellaCaporiacco, 1951 is a monotypic genus, described with a specimen whose type is possibly lost and which we presume is a juvenile. In this sense, the genital morphology of the species is unknown. The external morphology of S. pizaiCaporiacco 1951 and its relative geographic proximity suggest a close relationship with Rhopalocranaus bordoniŠilhavý, 1979. Both share characteristics such as the ornamentation of the mesotergal areas (presence of yellow tubercles and size of the spines), which distinguish them from Rhopalocranaus marginatus Roewer, 1913, type-species of the genus.
The penis of R. bordoni was illustrated in Šilhavý (1979); however, due to our lack of knowledge about the genital morphology of the R. marginatus, a comparison that allows us to make a decision on the taxonomic status of Stygnicranella in relation to Rhopalocranaus is impossible in this moment. So, we decided to keep both species treated here in Rhopalocranaus until a study of the genital and comparative morphology of both groups is available.
Rhopalocranaus bordoniŠilhavý, 1979 (Figs. 15, 17)
Rhopalocranaus bordoniŠilhavý [Jun.] 1979: 326, figs. 11-14.
Rhopalocranaus bordoni: Rambla and Juberthie, 1994: 221.
“Mendellinia”bordoniAvram and Soares [Dec.] 1979: 92, figs. 22-26. Syn. nov.
“Mendellinia”bordoni: Soares and Avram, 1987: 78, fig. 56; Rambla and Juberthie 1994: 221.
Holocranaus bordoni: Kury, 2003: 93.
Taxonomic summary
Type data: Rhopalocranaus bordoni: Venezuela, Miranda, Cueva Alfredo Jahn [10.485236° -66.242806°], ♂ holotype, 1♂ 1♀ paratypes (MHNG). Medellinia bordoni: Venezuela, Miranda, Virongo [Birongo]: Cueva A. [Alfredo] Jahn, ♀ holotype (MZTU).
Distribution: Cordillera de la Costa montane forest (NT0117) ecoregion (Fig. 17).
Remarks
Pierre Strinati and Carlos Bordón collected specimens from which a female was studied by Avram and Soares, while the rest of the material was studied by Šilhavý. Due to this, subsequent multiple synonyms resulted, as those of the Czech author and the Romanian-Brazilian team, who published both their species only months apart.
This synonymy was undetected for a long time due to multiple factors: 1) Šilhavý chose to include this species in Rhopalocranaus, a genus placed in the synonymy of Cranaus Simon, 1879 (then in Gonyleptidae Cranainae) 3 decades before by Soares and Soares (1948: 593), but without revalidating it. This usage of this invalid genus without any comment or formal revalidation had been done before by Schenkel (1953: 55) who also described a new Rhopalocranaus from Venezuela, and would be done later by González-Sponga (1991: 205) who redescribed Rhopalocranaus albilineatus Roewer, 1931. Rhopalocranaus was only formally revalidated much later by Kury (1997a: 4), who also transferred this genus to the Manaosbiidae. 2) Avram and Soares chose to place their new species in the then monotypic Colombian genus Medellinia Mello-Leitão 1939 (which they incidentally misspelled as “Mendellinia”, probably mixing up the city of Medellín with the surname Mendel/Mendes/ Méndez), now known to be a bona fide cranaid (Kury, 2003; Villarreal, 2016). 3) Medellinia was included in the synonymy of Holocranaus Roewer, 1913 by Kury (2003: 93) in the Cranaidae. At the same time, Avram’s M. bordoni was combined into Holocranaus (rather as a quick nomenclatural solution, not intended to be an informed taxonomic decision), being thus buried into another family.
Now, a careful restudy of the original description, and study of material (unfortunately burned in the 2018 fire in Museu Nacional) allows us to establish this synonymy, as both alleged species coincide in all fine details, even tarsal counts, and body/appendage measurements.
Rhopalocranaus flaviaculeatusCaporiacco, 1951 (Figs. 16-18)
Rhopalocranaus flaviaculeatusCaporiacco, 1951: 28, fig. 15.
Cranaus flaviaculeatus - Kury, 2003: 92.
Stygnicranella pizaiCaporiacco, 1951: 24, fig. 13; Kury, 1995a: 31. Syn. nov.
Taxonomic summary
Type data: Rhopalocranaus flaviaculeatus: Venezuela, Distrito Federal, El Junquito (10.46118° -67.080764°), ♀ holotype (MBUCV 478) (lost, Rubén Candia pers. comm. 2009). Stygnicranella pizai: Venezuela, Distrito Federal, El Junquito, juv. holotype (MBUCV 471) (lost, Rubén Candia pers. comm. 2009).
New record: Aragua: Colonia Tovar (Fig. 16A) and Rancho Grande, Parque Nacional Henri Pittier (Figs. 16B, 18C, D). Photographic records.
Distribution: cloud forests (Fig. 18C, D) of Cordillera de la Costa montane forest (NT0117) ecoregion (Fig. 17).
Remarks
Stygnicranella pizai was originally described in Stygnicranainae (Caporiacco, 1951), due to having very long pedipalps. Kury (1995) removed the name from Stygicranainae and placed it in Cranainae. Here, we hypothesize that a juvenile male of the family Manaosbiidae, based on the color pattern that is quite coincident with the juveniles of R. flaviaculeatus, and both being from the same locality. Unfortunately, the types of these species are apparently lost (Ruben Candia 2009, pers. comm).
Discussion
Published data of geographic distribution apparently indicate that the Neotropical fauna of Opiliones have a high rate of endemism (Arroyo-Peres et al., 2017; Pinto-da-Rocha et al., 2005), nevertheless, one must take into consideration that most data available come from single collecting events. There are only a handful of papers with structured collecting efforts over broader areas. More studies of this kind could further prove this trend but also show that it is not uncommon for species to have wider distributions, especially in large regions with less environmental heterogeneity. However, at least 2 recent studies found a broader distribution than assumed for species of harvestmen in Venezuela and Guyana, and drew attention to improve the knowledge of some of the species described for these countries (García et al., 2017; García & Kury, 2020).
We lack the knowledge to estimate how many kinds of distributional patterns can be recognized for harvestmen. There seem to be at least 2 main opposite patterns, which should be carefully evaluated when proceeding with balanced taxonomic decisions: 1) “widespread” species, which have distributions covering hundreds of kilometers (e.g., Anduzeia punctatum (Sørensen, 1932) (González-Sponga, 1992), Acritas bilineatus Sørensen, 1932 (García et al., 2017), Acropsopilio chilensis Silvestri, 1904 (Maury et al., 1996), the latter even crossing numerous national borders. This is a dangerous taxonomic pitfall. Sometimes, authors failed to recognize distant populations of the same species based on subtle local variations. This led them to describe specimens from each locality as new species. Examples of this are: Neocynorta venezuelensis (Roewer, 1915), described under 4 names by 3 authors independently (see Medrano et al., 2019); Phareicranaus curvipes (Roewer, 1916), described 5 times by 4 authors or Phareicranaus albilineatus, described 4 times by 2 authors (see González-Sponga, 2003; Pinto-da-Rocha and Bonaldo, 2011 and herein). 2) Short-range endemic (SRE) species, which display restricted geographic distributions, nominally less than 10,000 km2 (Harvey, 2002), then appear to occur on almost every mountain top, island, or in almost every isolated patch of forest (Jay et al., 2016). The Venezuelan genus Neocynorta Roewer, 1915 recently reviewed by Medrano et al. (2019) shows a pattern of several species (except for N. venezuelensis) occurring along a mountain chain, separated by a few tens of km from each other. A similar pattern was also found for the genus Eutimesius Roewer, 1913, with some SRE Andean species (except E. simoni Roewer, 1913 a widespread Amazonian species) and at least about 10 undescribed species from patches of Andean forest in both Colombia and Venezuela (Villarreal O., unpublished data) and the Brazilian genus Trasychiroides Soares & Soares, 1947, with 4 nominal species, each endemic to mountain tops of southern and southeastern Brazil (Pinto-da-Rocha et al., 2014)
On the other hand, there is a recurring pattern of species distributed in the Venezuelan Bolívar state and in Guyana, generating thus a potential taxonomic trap only because national borders are crossed. This pattern is exemplified by: 1) scorpiones. Ananteris venezuelensis G. S., 1972 and Tityus clathratus C. L. Koch, 1844 (Lourenço, 2012, 2013), although, this last species has a slightly wider distribution, reaching the Cordillera de la Costa in Venezuela (Moreno-González et al., 2019). 2) Birds. Atlapetes personatus (Cabanis, 1849); Poecilotriccus russatus (Salvin and Godman, 1884); Quiscalus lugubris Swainson, 1838 (Lepage, 2020). 3) Frogs. Oreophrynella quelchii Boulenger, 1895; Tepuihyla warreni (Duellman and Hoogmoed, 1992), recorded in Guyana and Venezuela (Villarreal et al., 2002).
Misplaced nationalism causes some authors to ignore any information on species beyond their country borders. It is common for authors with such a regional mindset to describe species as new just because they are new records from their country (e.g., Strand, 1900 for Norway; Morin, 1934 for Ukraine; Mkheidze, 1952 for Georgia). González-Sponga offered a prime example of this, by almost exclusively looking at Venezuelan specimens, overlooking precious information about neighboring countries. He even recommended that foreign authors should not work on Venezuelan fauna (González-Sponga, 2003: 67) because they “lack knowledge of the country’s geography” and because they “poach material, taking them to foreign museums”.
While we partially agree with González-Sponga’s considerations (the part of taking taxonomic decision without considering the geography, and the importance of part of the material being deposited in local collections, promoting their development), we stress here that his view allied with blindness to outside-of-the-country information is deleterious to taxonomy. This is what happens, for example, to India, which along with Venezuela is in the top-10 countries of the world with the most diverse opilionofauna (Kury, HarvEx, unpublished data), but which has been closed for international cooperation for decades, hindering immensely the advancement of taxonomy there.











 nueva página del texto (beta)
nueva página del texto (beta)



