Servicios Personalizados
Revista
Articulo
Indicadores
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Boletín de la Sociedad Botánica de México
versión impresa ISSN 0366-2128
Bol. Soc. Bot. Méx no.84 México jun. 2009
Taxonomía y florística
Floristic differentiation in limestone outcrops of southern Mexico and central brazil: a beta diversity approach
Diferenciación florística en afloramientos calizos del sur de México y el centro de Brasil: un enfoque de diversidad beta
Eduardo A. Pérez–García1,3, Anderson C. Sevilha2, Jorge A. Meave1 and Aldicir Scariot2
1 Departamento de Ecología y Recursos Naturales, Facultad de Ciencias, Universidad Nacional Autónoma de México, Circuito Exterior s/n, Ciudad Universitaria, México, D.F. 04510, México. 3 Corresponding author. E–mail: eduardo.perez–garcia@ciencias.unam.mx
2 Laboratório de Ecologia e Conservação, EMBRAPA Recursos Genéticos e Biotecnologia (CENARGEN). Brasília, D.F., Brazil.
Received: August 13, 2008.
Accepted: March 3, 2009.
Abstract
We studied the spatial arrangement of floristic diversity in two systems of limestone outcrops, located in two distant Neotropical sites: the region of Nizanda (S Mexico) and the Paranã Valley (Central Brazil). We addressed the question whether their vegetation could display a similar zonation, and we explored the relative effects of distance and an environmental gradient on α–, β– and γ–diversities. The limestone outcrops at both sites are similar in size and in elevation, but strongly differ in between–outcrop distance by an order of magnitude. At each study site three individual limestone outcrops were selected; in each of them three plant communities along the edaphic gradient were distinguished (a xerophytic scrub and two tropical dry forests types, one of which had a more xeric character than the other), and sampled for structural variables and floristic composition in 100–m2 plots. At both study sites, structural variables responded similarly to the edaphic gradient. Species density was larger in Nizanda for both α– and γ–diversity, but the largest value of β–diversity was obtained in Paranã. The edaphic gradient produced larger mean β–diversity values than the simple distance effect, with the interaction of both factors resulting in an even larger β–diversity. Classification analyses by site showed larger floristic similarities between the two xerophytic communities than those existing between them and the more mesic forests. The spatial arrangement of diversity showed that both α– and γ–diversity were smaller for the xerophytic communities. As hypothesized, the more extreme changes in community physiognomy were associated with larger β–diversity values.
Key words: Alpha diversity, gamma diversity, rupicolous plants, seasonally dry tropical forest, species turnover, xerophytic scrub.
Resumen
Estudiamos el arreglo espacial de la diversidad florística en dos sistemas de afloramientos de roca caliza localizados en dos sitios neotropicales distantes entre sí: la región de Nizanda (sur de México) y el valle del río Paranã (centro de Brasil). Los objetivos fueron analizar si la zonificación de la vegetación de estos dos sistemas era similar y explorar los efectos relativos de la distancia y de un gradiente ambiental sobre las diversidades α, β y γ. Los afloramientos calcáreos en ambos sitios son similares en tamaño y altitud, pero las distancias entre ellos difieren fuertemente entre las dos regiones por un orden de magnitud. En cada sitio seleccionamos tres afloramientos distintos y en cada uno de ellos distinguimos tres comunidades vegetales ubicadas a lo largo de un gradiente edáfico (un matorral xerófilo y dos tipos de bosque tropical, uno de ellos con un carácter más xérico que el otro), y las muestreamos usando parcelas de 100 m2 para estimar variables estructurales y conocer su composición florística. En ambos sitios las variables estructurales respondieron de manera similar al gradiente edáfico. La densidad de especies fue mayor en Nizanda tanto para la diversidad α como para la γ, pero la diversidad β más grande se obtuvo en Paranã. El gradiente edáfico produjo valores promedio de diversidad β mayores que los asociados al simple efecto de distancia, pero la interacción de ambas variables generó una diversidad β aún mayor. Los análisis de clasificación por sitio mostraron que la similitud florística es mayor entre las comunidades xerófilas que entre éstas y los bosques más mésicos. El arreglo espacial de la diversidad mostró que tanto la diversidad a como la y fueron menores en las comunidades xerófilas. Los resultados apoyan nuestra hipótesis de que los cambios más extremos en la fisonomía de estas comunidades están asociados con los valores más grandes de diversidad β.
Palabras clave: Diversidad alfa, diversidad gamma, matorral xerófilo, plantas litófitas, recambio de especies, bosque tropical estacionalmente seco.
A major goal in conservation biology is the protection of the largest possible number of species in areas that sometimes are relatively small. To this aim, knowing the spatial arrangement of biodiversity may be crucial (Whittaker, 1960; Arita and Rodríguez, 2001; Halffter et al., 2005), since a large gamma (γ)–diversity (i.e. total species richness in a region or in a set of individual study units, totaling a given area) may result from different combinations of alpha(α)–diversity (i.e. number of species in a locality or in one individual study unit forming part of a set of many similar units, each representing a fraction of the total area), and beta (β)–diversity (a measure of the difference between sites in a region or between study units) (Whittaker, 1960; Felfili and Felfili, 2001; Magurran, 2004; Pérez–García et al., 2005). This issue is particularly important in the dry tropics because of their large vegetational heterogeneity observed at various spatial scales, usually associated to a sizeable floristic variability (Pérez–García et al., 2005).
In the tropical dry regions of the world, mainly characterized by a climate of the AW type, two major plant formations have been recognized (Walter, 1973; Murphy and Lugo, 1986; Mooney et al., 1995; Cox, 2001; Trejo and Dirzo, 2002; Pennington et al., 2004; Prance, 2006). These plant formations are called tropical grasslands (variously known as savanna, cerrado, llanos, vegetación sabanoide), and tropical dry forest (seasonally dry tropical forest, tropical deciduous forest, selva baja caducifolia, or caatinga, among other names). Also, within each region dominated by either one of these formations, it is possible to distinguish a gamut of plant communities (usually recognized as vegetation types, plant associations, phytophysiognomies, etc.), which are linked to environmental factors like topography, surface lithology, soil depth, water and nutrient availability, and disturbance regimes (Walter, 1973; Silva et al., 1996; Balvanera et al., 2002; Durán et al., 2006; Gallardo–Cruz et al., in press.). Actually, much of the variation of these factors is perceived by plants as differences in water availability (Martínez–Yrízar et al., 2000). Consequently, landscapes located in regions dominated by tropical dry forest or savannas tend to be more heterogeneous than those located in tropical rain forest regions (see compilation by Oliveira and Marquis, 2002).
The prevailing plant communities in the forested tropical region have been encompassed under the term tropical dry forest (TDF, Murphy and Lugo, 1986). TDF is usually applied to forests of low elevation (< 1,400 m a.s.l.) from intertropical regions with a well defined dry season (Mooney et al., 1995; Gentry, 1995). The length of the dry season is highly variable, although it normally lasts for between four and six months. In TDFs total annual precipitation generally ranges from 500 to 1,800 mm (Gentry, 1995; Trejo, 1996, 1998), sub–zero temperatures are lacking, and their minimum mean annual biotemperature ranges between 18 and 26°C (Murphy and Lugo, 1995; Trejo, 1996, 2005). Despite these generalities, it must be acknowledged that the circumscription of TDFs is not easy. For example, establishing its boundaries in terms of the presence of foliage in plants during the dry season is troublesome, as the limits between varying degrees of deciduousness are arbitrary and difficult to assess in the field (Martínez and Galindo–Leal, 2002; Scariot and Sevilha, 2005). A further complication in setting the limits to the concept of TDF is the gradually increasing climatic seasonality of a tropical region as one moves away from the equator. However, even for the most equatorial tropical systems it is generally possible to distinguish between a humid (moist/rain) forest and a dry (deciduous) forest. Such differentiation is based on the lower stature of the canopy trees (< 30 m, but usually much less) and the degree of deciduousness of the dry forests (> 75%), along with other characteristics, such as the lower diversity and abundance of epiphytes in the TDF.
In addition to TDF, the world's tropical dry regions are notorious for encompassing various vegetation types (Menaut et al., 2005; Lott and Atkinson, 2006), even in small areas, due to local variations in water and nutrient availability (Sampaio, 1995; Pérez–García et al., 2001). Such variability involves a complex array of plant communities, some of which are equally elusive of a simple definition (Eiten, 1986; Furley, 2006), ranging from dense, large biomass and relatively tall riparian forests, through several types of savanna and deciduous scrub, to open, sparse, xerophytic vegetation typical of rock outcrops. Among the latter, the floristic composition of the vegetation occurring on limestone outcrops is of special interest, as it contains a considerable share of endemic species that are absent from the surrounding areas (Borhidi, 1996; Silva and Scariot, 2003, 2004a, b; Pérez–García and Meave, 2004).
Despite the biological importance and the particular ecological conditions in systems of limestone outcrops, few studies have examined their characteristics in detail. An example is found in the work of Borhidi (1996), who described seven plant associations on limestone outcrops (mogotes) in Cuba. He distinguished two different mogote forest types: one typical of the deeper soils of piedmonts, and the other growing on bare rock. Besides, among non–forest vegetation types he recognized monocot–dominated (with Bromeliaceae and Agave), and shrub–dominated communities. The presence of succulent plants, such as Agave and columnar or liana–like cacti, epiphytes, and water–storing trees with barrel–shaped trunks are characteristic of the extremely dry and hot habitats of these limestone outcrops. A similar differentiation in vegetation type was found by Pérez–García and Meave (2004) in limestone outcrop systems of the Isthmus of Tehuantepec region (S Mexico). They described three different plant communities based on their physiognomy; two of them corresponded to different types of forest communities possessing a continuous canopy, and a xerophytic scrub with a discontinuous plant cover. The differentiation between the two forest communities was based on the fact that each is associated to a different substrate; one of them grows on the exposed limestone bedrock, and the other develops on continuous and deeper soils. Preliminary field observations conducted in the Paranã River basin showed an analogous spatial structure of the vegetation.
Restrictions for plant growth, together with the consequent ecological specialization of the rock outcrop flora, are well documented phenomena (Silva et al, 1996; PavletiC and Trinajstic , 1997; Porembski et al, 1998; Barthlott and Porembski, 2000; Seine et al., 2000). These constraints on plant growth are related to a discontinuous soil cover and a small soil volume, a combination that causes lower water and nutrient availability. Additionally, the scarce plant cover allows more solar radiation to reach the ground, increasing soil temperature and evaporation rates. overall, moving from lower topographic positions to the hilltops of limestone outcrops strong decreasing gradients of soil humidity and fertility are found.
A further feature that makes TDF of the Americas of particular interest is that, unlike those forests typical of the almost continuous humid areas that span from Amazonia to the Mexican Atlantic watershed (Hartshorn, 2002), TDF regions are more discontinuous (figure 1), and the largest areas of this vegetation type are geographically very distant (Miles et al., 2006). Habitat continuity allows species to disperse across large distances of biogeographic scale (cox, 2001). This is relevant because it appears that Meso–american tropical rain forests represent a relatively recently evolved floristic subset derived from the Amazonian species pool (Wendt, 1998), although for the TDF this does not appear to be the case, as this forest type may have a diverging evolutionary history (Gentry, 1995; Cevallos–Ferriz and Ramírez, 2004; Becerra, 2005).
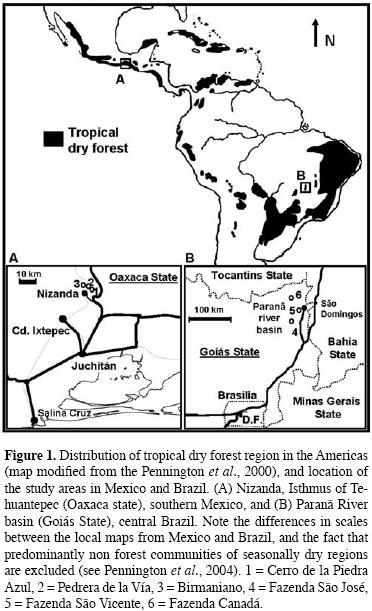
Due to the biogeographic discontinuity of the TDF region throughout the Americas, plant communities located at opposite ends of its geographical range hardly share any species; this differentiation is particularly evident for mature forest species (Lott and Atkinson, 2006). Given such transcontinental floristic differentiation, it is unclear whether systems of limestone outcrops located far–apart within this region display similar floristic ensembles, and by analogy, if the spatial distribution of species and community attributes results in an equivalent vegetation zonation. The study of such patterns may be useful in exploring the existing hypotheses about β–diversity. Therefore, having identified two distant systems of limestone outcrops in the Americas (southern Mexico and central Brazil), our goal was to make a comparison between them in order to answer the following questions: What is the contribution of α– and β–diversities to γ–diversity in these limestone outcrops, and which factor, i.e. the edaphic gradient or the spatial separation (distance), is more strongly associated with a larger floristic differentiation?
Based on the environmental structure typical of limestone outcrops described above, we hypothesized that, in comparing sets of vegetation samples, larger differences in physiognomy would be associated to a large floristic turnover (β–diversity). In turn, due to the limitations of dispersal over large distances, it was reasonable to expect that floristic turnover would be even greater with increasing between–sample distances.
Material and methods
Study areas. The study was conducted in two Neotropical localities of the seasonally dry forest region located more than 6,000 km apart (figure 1). In both localities a conspicuous terrain feature is the presence of limestone outcrops (Lo), which are nearly half a kilometer long and usually no less than 200 m wide.
Nizanda (Oaxaca), Mexico.– The site is located on the Pacific slope of the Isthmus of Tehuantepec (figure 1). The regional climate is highly seasonal, warm and sub–humid, with a mean annual temperature of 26°C. Annual precipitation (mean ca. 1,000 mm) concentrates from August to November (Pérez–García and Meave, 2004). A published vascular plant checklist for this region includes 746 species (Pérez–García et al., 2001), although an updated inventory comprises over 920 species. The prevailing vegetation type is a low stature, tropical dry forest (selva baja caducifolia, after Miranda and Hernández–X., 1963), with a mean canopy height of ca. 7 m. Maximum elevation at the three studied LO ranges between 250 and 350 m a.s.l., and they are separated from each other by narrow ravines cut by seasonal streams (Pérez–García and Meave, 2004).
Paranã River basin, Brazil.– The location of this site is central Brazil, Goiás state (figure 1). The Paranã River basin (hereafter Paranã only) has a S to N orientation, and its elevation ranges from 400 to 600 m a.s.l. Regional climate is warm, with a mean annual temperature of 24°c, and an average total annual precipitation of 1,500 mm (Silva and Scariot, 2003 , 2004a, b). Rainy season concentrates between spring and summer (October to March) (Scariot and Sevilha, 2005).
Seasonally dry tropical forest (floresta estacional decidua or mata seca) is the predominant natural vegetation type, with a canopy height of around 20 m; this community may thus be classified as medium–height tropical deciduous forest sensu Miranda and Hernández–X. (1963).
The three LO studied at Paranã form part of São Domingos municipality (Goiás State), and are located in different cattle farms: Fazenda São José (Silva and Scariot, 2004a), Fazenda São Vicente (Silva and Scariot, 2004b), and Fazenda Canadá (Silva and Scariot, 2003).
All studied LO are well conserved, which is certainly due to their rocky nature and to their very irregular topography. In contrast, there is one major difference between the two systems regarding the spatial separation between the LO. The largest between–limestone outcrop distance in the Mexican locality is ca. 2 km, whereas outcrops in Brazil are separated by distances as large as 84 km. For the two systems previous plant surveys are available (Silva and Scariot, 2003, 2004a, b; Pérez–García and Meave, 2004), but for the Brazilian LOs the floristic checklists include trees only. In the LO of Brazil, all plant with DBH > 5 cm were recorded in 400–m2 plots. For the LO of Fazenda Canadá 924 trees were found, and they belong to 24 families, 38 genera and 48 species (Silva and Scariot, 2003). In Fazenda São Vicente the survey produced a total of 860 living and 36 dead trees, and they represented 51 families, 41 genera and 51 species (Silva and Scariot, 2004b). Finally, for Fazenda São José, a total of 536 living and 52 dead trees were recorded, and the trees belonged to 36 species, 31 genera and 21 families (Silva and Scariot, 2004a).
Vegetation sampling. In each individual limestone outcrop at both localities we distinguished and sampled three plant communities. The first one occurs on the piedmonts where soil is continuous and relatively deep (> 50 cm), and was referred to as tropical dry forest on soil (TDFs). Moving up along the topographic gradient a second forest type is found, which is established on the exposed limestone matrix; it was named tropical dry forest on bare rock (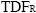 ). According to Pérez–García and Meave (2004), these two forests are not only floristically different (the TDFs being richer in species), but they also differ in their physiognomy, such as a larger occurrence of trees with water–accumulating stems in the
). According to Pérez–García and Meave (2004), these two forests are not only floristically different (the TDFs being richer in species), but they also differ in their physiognomy, such as a larger occurrence of trees with water–accumulating stems in the 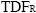 . The third community, restricted to outcrop tops and escarpments with exposed limestone, was classified as xerophytic scrub (XS) due to its discontinuous canopy and the low stature (< 4 m) of the woody stratum (figure 2).
. The third community, restricted to outcrop tops and escarpments with exposed limestone, was classified as xerophytic scrub (XS) due to its discontinuous canopy and the low stature (< 4 m) of the woody stratum (figure 2).
In terms of environmental harshness, there are indications of the existence of an increasing gradient of xerophytic conditions in these plant communities as: TDFs < 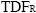 < XS. For concision, the
< XS. For concision, the 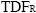 and XS will be hereafter referred to together as xerophytic communities, whereas
and XS will be hereafter referred to together as xerophytic communities, whereas 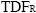 and TDFs will be referred to as forest communities. This means that
and TDFs will be referred to as forest communities. This means that 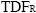 is included in both the xerophytic and the forest communities (Pérez–García and Meave, 2004). In the studied limestone outcrops the two forest communities covered larger and more continuous areas, while the xerophytic scrub had a patchier and limited distribution. The small extent of this latter community determined the use of small–sized sampling units.
is included in both the xerophytic and the forest communities (Pérez–García and Meave, 2004). In the studied limestone outcrops the two forest communities covered larger and more continuous areas, while the xerophytic scrub had a patchier and limited distribution. The small extent of this latter community determined the use of small–sized sampling units.
Vegetation was sampled in 10 × 10 m plots, in which we recorded all individuals > 1 cm DBH rooted within the plot; this set was referred to as upper stratum. A lower stratum was sampled in five 2 × 2 m subplots placed at each corner and in the centre of the plot; this stratum included woody plants and herbs > 30 cm tall, but < 1 cm DBH, globose cacti > 5 cm in diameter, and terrestrial rosettes and colonial plants (individual or colonial cover > 20 cm in diameter). In Paranã, only one plot per vegetation type was taken from each limestone outcrop, which resulted in a total of nine plots (1 plot by community × 3 communities × 3 limestone outcrops), whereas in Nizanda three plots were taken by community, giving 27 plots in total (3 plots by community × 3 communities × 3 limestone outcrops). Within these plots, the height and two perpendicular diameters (from which cover was calculated later) of each individual plant were measured, and its identity recorded; for the upper stratum plant's DBH was also measured. The high frequency of clonal plants in the lower stratum prevented the counting of individuals and therefore density values were calculated for the upper stratum only.
Based on the most frequent species per community and per site, we identified potential ecologically equivalent species. To this end we considered both species actually encountered in our samples and those reported in the published checklists (Pérez–García et al., 2001; Silva and Scariot, 2003, 2004a, b; scariot and sevilha, 2005). Ecological equivalence was assessed according to similar habitat preferences, taxonomic identities, and morphological attributes. Among the latter, we took into consideration the natural variability in the vegetation of the two study systems, as trees in Paranã may be twice as big as those at Nizanda. in addition, we used morphological criteria such as plant height, leaf morphology, presence of spines, and occurrence of succulent stems.
Data analyses. A binary (presence/absence) data matrix of species distribution for each of the two studied sites was constructed. With these matrices we were able to arrange the samples in several ways in order to evaluate the effect of plot location on species density and the β–diversity in these systems. Because vegetation sampling at each site involved three different communities and three separate Lo, samples were taken in groups of three plots (which we named triplets). Each triplet included one of three possible combinations. The first combination included three samples from the same community, but from different Lo; this combination minimized environmental effects, but maximized the effect of distance. Next, a different combination included one sample from each community, but all from the same Lo; this combination maximized the effect of environmental heterogeneity, but minimized the effect of distance. The third combination consisted of one sample from each community, each from a different Lo; unlike the previous combinations, this one maximized both the effects of environmental heterogeneity and of distance. These combinations were selected to test the hypothesis that β–diversity would increase from the first to the third triplet combination.
For each triplet we calculated Whittaker's β–diversity index (Pw, Whittaker, 1960; Wilson and Shmida, 1984), as follows:
βW = γ/α
where γ is total number of species for the triplet, and α is mean species density per plot. indices were calculated only for plots located in the same site, but not for groups involving both Mexican and Brazilian plots.
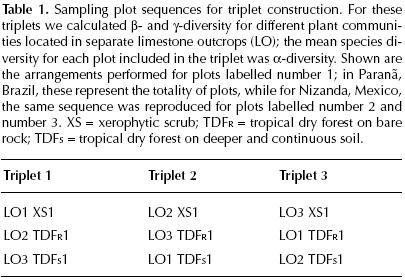
In order to evaluate a distance–related effect on βW, we calculated this value for each triplet of plots from the same community (either TDFs, 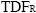 or XS), but with each plot coming from a separate limestone outcrop, i.e. first triplet combination described above (figure 3, exemplified by plots labeled with number 1). Because of the limited sampling intensity in Paranã (only three plots per community), we were able to calculate βW only once for each community. in contrast, the larger number of plots per community in Nizanda enabled us to obtain several values for PW, which allowed calculating a mean (± 1 S.E.) value (in the tables of Results the number of triplets used for its calculation is indicated). In all cases, each plot was used only once in calculating each value of this index.
or XS), but with each plot coming from a separate limestone outcrop, i.e. first triplet combination described above (figure 3, exemplified by plots labeled with number 1). Because of the limited sampling intensity in Paranã (only three plots per community), we were able to calculate βW only once for each community. in contrast, the larger number of plots per community in Nizanda enabled us to obtain several values for PW, which allowed calculating a mean (± 1 S.E.) value (in the tables of Results the number of triplets used for its calculation is indicated). In all cases, each plot was used only once in calculating each value of this index.
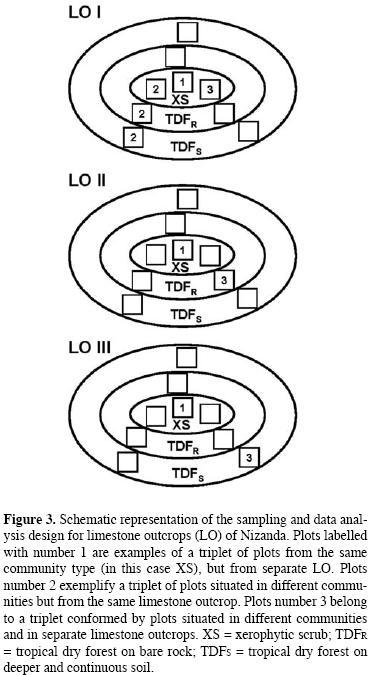
Similarly, to evaluate the effect of the environmental gradient on βW we used triplets of plots each from a different community, but all from the same LO, i.e. second triplet combination described above (figure 3, exemplified by plots labeled number 2). In addition, we assessed the combined effect of distance and environmental gradient on βW, which required using triplets of plots both from different communities and different LO, i.e. the third above–described combination (figure 3, exemplified by plots labeled number 3). Again, plots were used only once in each calculation. The smaller number of plots in Brazil prevented us from comparing the spatial configuration of diversity within the same community and the same LO.
The sampling plots of Nizanda were numbered 1 to 3 for each community. In the comparisons between LO or between communities, only plots bearing the same number were compared. The basic scheme of triplet construction is illustrated in table 1; the arrangement shown includes all of the Paranã samples, while it exemplifies the set of triplets containing all plots numbered 1 for Nizanda. For this latter site, subsequent calculations of βW were done by using the same numerical sequence of plots, but changing the plot number.
Discrimination of the magnitude of the effects of distance and the environmental gradient in limestone outcrops required examining the between–site differences of mean βW values calculated for different sets of triplets. Because absolute differences may not be comparable as they would reflect the magnitude of the original βw values rather than that of their difference, we chose to express these differences as percentages, as follows:
% difference = [1 ( (βWS / βWL)] × 100
where βWS is the smaller and βWL is the larger of the values being compared. According to this procedure, estimated percent differences were always either positive or zero when the two compared values of βW were identical.
We performed numerical classifications of the samples of each site using Ward's (minimum variance) clustering method, with Euclidean distances as measure of dissimilarity for the binary data matrix (Kent and Coker, 2003), using the STATISTICA software (StatSoft Inc., 1995).
Results
Quantitative structure. Total number of recorded species in the twenty seven 100–m2 plots from Nizanda was 211, whereas in the nine equal–sized plots from Paranã total richness was 78. At both study sites, TDFs had the largest mean species density, and this was always followed by 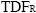 , with the lowest values corresponding to XS (table 2). Over–all, plots from Nizanda were more diverse than those from Paranã, but this was particularly noticeable for the two xerophytic communities (i.e. the
, with the lowest values corresponding to XS (table 2). Over–all, plots from Nizanda were more diverse than those from Paranã, but this was particularly noticeable for the two xerophytic communities (i.e. the 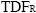 and the XS). In contrast, the partitioning of species density values by stratum (upper and lower) did not produce any clear pattern, neither between sites nor between communities. Mean contribution of the upper stratum to total species density by plot was 60%, while the contribution of the lower stratum was 57%. These percentages mean that on average each stratum harbors exclusively ca. 40% of the total species density, implying a strong between–strata complementarity (sensu Vane–Wright et al., 1991).
and the XS). In contrast, the partitioning of species density values by stratum (upper and lower) did not produce any clear pattern, neither between sites nor between communities. Mean contribution of the upper stratum to total species density by plot was 60%, while the contribution of the lower stratum was 57%. These percentages mean that on average each stratum harbors exclusively ca. 40% of the total species density, implying a strong between–strata complementarity (sensu Vane–Wright et al., 1991).
Total plant cover was lowest in XS. This result is consistent with the initial physiognomic distinction between communities. According to such distinction, which is based on the occurrence (or lack thereof) of a continuous tree cover, no clear differences were expected between the two forest types. However, percent cover in TDFs was in both sites 25–33% larger than that of 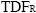 , implying that in absolute terms in the TDFs there is virtually an additional layer of foliage (100% extra of plant cover; table 2). As expected, in both forest types and at both sites, the upper stratum made the largest contribution to total cover. The similarity between total cover of TDFs in the two sites was remarkable (405% and 407% for Nizanda and Paranã, respectively), which was not the case for Xs, as the value obtained for Nizanda was 1.6 times that obtained for Paranã (table 2). Similar decreasing trends from TDFs to XS were observed for basal area and density, with two important exceptions: a much larger basal area (0.66 m2/100 m2) for the
, implying that in absolute terms in the TDFs there is virtually an additional layer of foliage (100% extra of plant cover; table 2). As expected, in both forest types and at both sites, the upper stratum made the largest contribution to total cover. The similarity between total cover of TDFs in the two sites was remarkable (405% and 407% for Nizanda and Paranã, respectively), which was not the case for Xs, as the value obtained for Nizanda was 1.6 times that obtained for Paranã (table 2). Similar decreasing trends from TDFs to XS were observed for basal area and density, with two important exceptions: a much larger basal area (0.66 m2/100 m2) for the 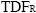 of Nizanda and a low density for the TDFs of Paranã. In fact, density values for both forests types in Paranã were considerably smaller than their Nizanda counterparts (table 2).
of Nizanda and a low density for the TDFs of Paranã. In fact, density values for both forests types in Paranã were considerably smaller than their Nizanda counterparts (table 2).
Floristic differences between the two studied systems. Due in part to environmental differences, but mainly to the large geographical separation between the two sites, there are practically no coincidences between the two systems at the species level (table 3). Tabebuia impetiginosa (Mart. ex Dc.) standl., is a noteworthy exception; this species is important in Paranã according to its frequency and structural contribution (silva and scariot, 2004a, b), and it is also a common tree in the TDFs of Nizanda (Pérez–García and Meave, 2004; Gallardo et al., 2005). some taxa were found in the outcrops of Paranã but not in those of Nizanda, although in the latter region they also occur but restricted to moister communities, such as riparian forests or higher–elevation moist forests. This is the case of the species Guazuma ulmifolia Lam., Celtis iguanaea (Jacq.) Sarg., and Ficus insipida Willd., and of the genera Aspidosperma, Astronium, Cecropia, and Sapium. in fewer cases some shared genera were frequent in the TDF of Nizanda, either on limestone outcrops or in other habitats: Casearia, Cordia, and Heliocarpus. No species were shared between the xerophytic communities of the two localities, although several genera were common to both, e.g. Hechtia, Cnidoscolus, Ficus, Jatropha, and Pseudobombax. Their corresponding species were taken as potential ecological equivalents, given the uniqueness of the habitats where they grow, as well as their morphological convergences (table 3).
Spatial arrangement of limestone outcrop diversity. same community, different limestone outcrop.– At the two sites, when triplets of plots located in the same community but in different LO were analyzed, XS plots had the lowest y–diversity (average for Nizanda: 21 species; total for Paranã: 15 species). Gamma diversity by triplet was intermediate in 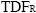 (average for Nizanda = 55.0; total for Paranã = 36), and highest in TDFs (average for Nizanda = 87.3; total for Paranã = 51). The same pattern was also true for α–diversity values in the three communities at the two sites (table 4).
(average for Nizanda = 55.0; total for Paranã = 36), and highest in TDFs (average for Nizanda = 87.3; total for Paranã = 51). The same pattern was also true for α–diversity values in the three communities at the two sites (table 4).
Between communities.– At the two study sites, mean γ–diversity by triplet for plots located in different communities (62.9 and 39.3 species in Nizanda and Paranã, respectively) was larger than the corresponding mean value for plot triplets sharing community (Nizanda = 54.4 and Paranã = 34 species). Particularly, γ–diversity for triplets of plots located in different communities was slightly larger for plots coming from separate LO than for those located in the same one (table 4).
The analysis of plot triplets located in different communities (i.e. along the edaphic gradient) and in separate LO produced larger mean β–diversity values (Nizanda = 2.60; Paranã = 2.73), than the corresponding ones for plots differing in community but not in LO (Nizanda = 2.54; Paranã = 2.63). All these values, including their averages, were larger than mean β–diversity obtained for triplets sharing community but differing in LO (Nizanda = 2.12; Paranã = 2.42; table 4).
Comparison of βW of triplets located in the same community with those located in a different one (but always from separate LO) allowed assessing the effect of environmental gradient upon this index. As expected, this comparison produced very large differences: for Nizanda the percent difference was 18.5% (βW = 2.60 for triplets differing in community vs. βW = 2.12 for triplets located within the same community), while in Paranã the percent difference was 11.4% (between the corresponding βW = 2.73 and βW= 2.42). These percent differences contrasted strongly with those obtained from the comparison of triplets always differing in community, but located either in the same or in a different LO. For Nizanda this percent difference was 2.31% (between respective values of βW = 2.60 and βW = 2.54 for triplets located in different and the same LO), and 3.7% for Paranã (between the corresponding βW values of 2.63 and 2.42). These results indicate that the effects of spatial separation are relatively small and of the same order of magnitude at both sites (< 1 percent points), whereas the effect of the edaphic gradient, despite being also of the same order of magnitude, was larger in Nizanda than in Paranã (> 7 percent points).
Classification analysis. The dendrograms resulting from the classification of plots based on their floristic composition showed two clearly distinct groups in the two study sites, at a linkage distance of 12 for Nizanda (figure 4), and of 7 for Paranã (figure 5). In both cases, one of these groups comprised all TDFs plots, and the other included all plots from the two xerophytic communities. The separation of the TDFs group is in full agreement with the original vegetation categorization; however, in neither case was it a very homogeneous group, as between–sample similarity was rather low (figures 4 and 5). In contrast, XS samples displayed larger similarities between them, and therefore they grouped at smaller linkage distances. For Nizanda, a partial differentiation between 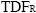 and XS samples was observed (figure 4), a situation which was not true in the case of Paranã (figure 5).
and XS samples was observed (figure 4), a situation which was not true in the case of Paranã (figure 5).
Discussion
Quantitative structure. The LO of Nizanda are characterized by a larger species density than those of Paranã, since they had not only a higher mean a–diversity, but also a larger mean y–diversity. The fact that diversity is larger at Nizanda is not surprising, because the Mexican TDF biome is recognized to include many highly diverse communities (Gentry, 1995; Trejo and Dirzo, 2002; Gallardo–Cruz et al, 2005). However, it was difficult to make an equally clear prediction for species density of XS and 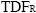 , given the scarce knowledge available for these two xerophytic communities. For Nizanda's XS we were able to estimate its total richness (74 species, including those shared with other communities; unpublished data). Also, for XS both a large diversity and a high structural importance of monocotyledons have been reported (Pérez–García and Meave, 2004); unfortunately we lack information to attempt similar comparisons with other localities.
, given the scarce knowledge available for these two xerophytic communities. For Nizanda's XS we were able to estimate its total richness (74 species, including those shared with other communities; unpublished data). Also, for XS both a large diversity and a high structural importance of monocotyledons have been reported (Pérez–García and Meave, 2004); unfortunately we lack information to attempt similar comparisons with other localities.
For the TDF of Paranã a complete regional species survey is not yet available, although there are indications that this region also harbors a large floristic diversity (total richness ca. 496; A. Scariot and A. Sevilha, unpublished data). In fact, the TDF that occurs on flat areas in the same region is more diverse than that growing on LO (Ivanausckas and Rodrigues, 2000; Scariot and Sevilha, 2005). The existing information on species richness for Paranã's LO refers basically to canopy trees (DBH > 5 cm; Silva and Scariot 2003, 2004a, b; Scariot and Sevilha, 2005). Therefore, we cannot estimate precisely the total species richness of this system, a limitation that is particularly true for xerophytic communities.
Some factors may explain the lower species densities of Paranã with respect to Nizanda. The first one is the much shorter distance from Nizanda to the sea, both to the Gulf of Mexico and the Pacific Ocean, a circumstance that results in a frequent exposure to humid winds at this locality (Romero–Centeno and Zavala–Hidalgo, 2003). This may be the reason for the profuse growth of soil–drought resistant plants, despite their vulnerability to atmospheric drought (e.g. some Bromeliaceae and Orchidaceae). The presence of such plants is frequent in LO of Cuba, which are relatively closer to the sea (Borhidi, 1996). Nevertheless, the proximity to the coast alone does not explain the abundance of epiphytes in the LO of Nizanda, as illustrated in the surrounding TDF beyond LO, or further west on the Oaxaca coast, near Huatulco, where a considerably taller TDF also hosts a very low epiphyte density and diversity (Salas–Morales et al., 2003).
A second explanation may be related to the fact that species richness is a function of the number of sampled individuals (Magurran, 2004), leading to the argument that the lower density observed in the TDFs of Paranã is responsible for its low richness relative to Nizanda. However, this explanation is not supported by our results, given that the 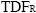 of Paranã had more individuals but less species than the TDFs in the same locality (table 2); in fact, a pattern of low species density in this forest type was reported for TDF all over the Paranã valley (Scariot and Sevilha, 2005).
of Paranã had more individuals but less species than the TDFs in the same locality (table 2); in fact, a pattern of low species density in this forest type was reported for TDF all over the Paranã valley (Scariot and Sevilha, 2005).
A third possible explanation may be related to the geographic location of Nizanda and its relative proximity to the large arid regions of central Mexico. This situation could be responsible for an abnormal increase in the number of xerophytic species occurring on the outcrops, a phenomenon also observed in some granite outcrops or inselbergs (Porembski et al, 1995). There is some evidence supporting this possibility, as illustrated by some xerophytic species such as Jatropha oaxacana JJiménez–Ram. et R.Torres, and Echeveria acutifolia Lindl., which are shared between the LO of Nizanda and the arid Tehuacán Valley in central Mexico. Nonetheless, the unequal floristic knowledge available for our study sites in Mexico and Brazil precludes drawing a definite conclusion about this factor.
The structural values calculated for all forest communities at both sites fall within the reported range for other TDF localities (Gentry, 1995; Trejo and Dirzo, 2002; Gallardo–cruz et al., 2005). The largest basal area was found in the 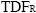 of Nizanda, but this figure could have been strongly influenced by plants capable of storing water in their stems (succulents), and thus it may not represent proper wood (Pérez–García and Meave, 2004). Therefore, the largest woody basal area may actually be that observed for the TDFs of Paranã, where the biggest trees were present. The recorded basal area in this study was larger than the value previously reported for these limestone outcrops as a whole, without distinction between communities (silva and scariot, 2003, 2004a, b), but the large value of Paranã recorded here was accounted for to some extent by a single individual of Cavanillesia arbórea (Willd.) K.Schum., having a DBH of 89 cm. This exemplifies the extent to which the presence of a succulent plant may result in a magnification of basal area.
of Nizanda, but this figure could have been strongly influenced by plants capable of storing water in their stems (succulents), and thus it may not represent proper wood (Pérez–García and Meave, 2004). Therefore, the largest woody basal area may actually be that observed for the TDFs of Paranã, where the biggest trees were present. The recorded basal area in this study was larger than the value previously reported for these limestone outcrops as a whole, without distinction between communities (silva and scariot, 2003, 2004a, b), but the large value of Paranã recorded here was accounted for to some extent by a single individual of Cavanillesia arbórea (Willd.) K.Schum., having a DBH of 89 cm. This exemplifies the extent to which the presence of a succulent plant may result in a magnification of basal area.
Spatial arrangement of floristic diversity. Overall, a larger average β–diversity was observed along the edaphic gradient at both sites, but in association with longer between–outcrop distances (i.e. in Paranã), species turnover was even larger. Moreover, the more extreme physiognomy (from the tallest vegetation to the most open xerophytic scrub) observed in Paranã coincided with the largest mean β–diversity (βW = 2.73). Nevertheless, it is noteworthy that the largest individual β–diversity value for a single triplet was found for the 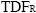 of Paranã (βW = 3.00). In Nizanda, this community is very heterogeneous in species composition (e.g. its mean β–diversity has the largest standard error). This variability encompasses dense thickets of the columnar cactus Neobuxbaumia scoparia (Poselg.) Backeb., patches of Plumeria rubra L., or of the lithophytic Ficus trees (Moraceae). Also, the associated arrays of understorey plants are very different, including mats of clonal monocots like Anthurium nizandense Matuda (Araceae), Agave nizandensis cutak (Agavaceae), Cyrtopodium macrobulbum (La Llave et Lex.) G.A.Romero (orchidaceae). unfortunately, we lack sufficient information to draw stronger conclusions about the very high β–diversity in the
of Paranã (βW = 3.00). In Nizanda, this community is very heterogeneous in species composition (e.g. its mean β–diversity has the largest standard error). This variability encompasses dense thickets of the columnar cactus Neobuxbaumia scoparia (Poselg.) Backeb., patches of Plumeria rubra L., or of the lithophytic Ficus trees (Moraceae). Also, the associated arrays of understorey plants are very different, including mats of clonal monocots like Anthurium nizandense Matuda (Araceae), Agave nizandensis cutak (Agavaceae), Cyrtopodium macrobulbum (La Llave et Lex.) G.A.Romero (orchidaceae). unfortunately, we lack sufficient information to draw stronger conclusions about the very high β–diversity in the 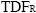 of Paranã.
of Paranã.
At the two sites, both α– and γ diversity were smaller for the xerophytic communities, particularly for xs. consequently, a clear pattern may be generalized of species reduction towards the more stressing environments. contrastingly, we did not find an equally clear pattern for β–diversity: only in Nizanda was there a reduction in β–diversity in the xerophytic communities, unlike the case of Paranã. Moreover, the observed values of α– and β–diversity were not clearly related with each other. Again, both in Nizanda and Paranã the percent differences in β–diversity associated to the distance effect were relatively small. This is particularly relevant because between–outcrop distances in Paranã were one order of magnitude larger than those at Nizanda. Apparently, small βW values related to geographical distance are common; for example, in the Brazilian "cerrado" β–diversity between adjacent communities is usually very low, and it is necessary to travel across huge distances before observing a significant floristic differentiation, except when soil differences exist (Felfili and Felfili, 2001). Nonetheless, in a TDF of western Mexico, Balvanera et al. (2002) found contrasting evidence as distance out–weighed the edaphic gradient. We may thus argue for the possibility of a tradeoff between distance and magnitude of the environmental gradient as determinants of β–diversity, although further information on the relative effects of both factors on between–community floristic differentiation is needed before drawing sounder conclusions.
Implications for conservation. The exclusive component of the xerophytic communities stands out given their particular morphological attributes and their high level of endemism (Torres–Colín, 1989; Burke, 2003; Pérez–García and Meave, 2004; silva and scariot, 2004a). The high level of endemism observed in the LO matches known patterns for Mexican arid zones (Rzedowski, 1962, 1991). For the LO of Nizanda several endemic species have been recorded, such as Agave nizandensis, Barkeria whartoniana (C.Schweinf.) Soto Arenas, Cephalocereus nizandensis (Bravo et T.MacDoug.) Buxb., Encyclia nizandensis Pérez–García et Hágsater, and Solandra nizandensis Matuda (Pérez–García et al., 2001). similarly, new species of Aspidosperma, Commiphora and Luetzelburgia were discovered recently in the LO of Paranã (scariot and sevilha, 2005). in the two dendrograms resulting from the classifications performed for both sites (figures 4 and 5), those mixed groups that include both samples from 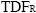 and XS demonstrate that these communities have more species in common than those shared by them with TDFs, despite the intermediate structural character of the
and XS demonstrate that these communities have more species in common than those shared by them with TDFs, despite the intermediate structural character of the 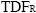 . Therefore, we may conclude that, rather than being an impoverished species subset of TDFs, the xerophytic component of the LO has it own identity.
. Therefore, we may conclude that, rather than being an impoverished species subset of TDFs, the xerophytic component of the LO has it own identity.
An important conclusion derived from this study is that for LO systems, or for other systems with similar spatial configurations of their diversity, species density may not necessarily be the best criterion to establish a protected area. Among the three communities studied by us, TDFs had the largest mean α– and γ–diversity values regardless the site, but these quantities were not matched by the largest βW value. In contrast, the mean larger β–diversity values were obtained from the comparison of communities with most dissimilar physiognomies. One implication of this finding, underlying the concept of β–diversity, is that the larger is β–diversity, the greater is the overall biological differentiation. Very limiting habitats sites are often poorer in species and thus make a smaller contribution to total γ–diversity of a region. However, the most specialized adaptations tend to concentrate in them, and they sometimes represent the only habitats for re–lictual species. Consequently, the incorporation of habitats into conservation schemes generally results in an increased diversity of life– and growth–form patterns, leaf morphology, ecological dispersal mechanisms, and ultimately, of evolutionary lineages (Chain–Guadarrama, 2005; López–Olvera, 2005; Pérez–García and Meave, 2006). Therefore, a conservation strategy uniquely aimed at maximizing species richness while disregarding species identities may be detrimental for the conservation of sets of species with an important biological value.
More studies providing detailed evaluations of the floristic similarities and differences between Neotropical LO are required. Particular interest should be paid to assess the role of these systems as seed sources, either for the regeneration of disturbed communities at present (Silva and Scariot, 2004b), or before a scenario of longer–term climatic change (see Pennington et al., 2000; Prance, 2006). In any case, the conservation of LO will rely upon the amount of knowledge that we can gather for these systems, since important attributes for their conservation, such as their large share of endemic species, may not be perceived at a first glance.
Acknowledgements
The Mexican Commission for Biodiversity (CONABIO–G018 and L085) and the National Council for Science and Technology (CONACYT–SEMARNAT–C01–0267) financed field work in Mexico and the first author's travel to Brazil. Field work in Brazil was supported by EMBRAPA–CENARGEN. P. Guadarrama, O. Núñez, N.F. Barbosa, A.R. Oliveira, and the people of Monte Alto and Nizanda, particularly Carmelito's and the Reyes–Manuel families helped during field work, and Y. Martínez, A. Bonesso Sampaio, M.A. Romero, and I. Sánchez–Gallén assisted in the laboratory work. CONACyT and DGAPA–UNAM awarded graduate scholarships to E.A.P.–G.
Literature cited
Arita H. and Rodríguez P 2001. Ecología geográfica y la macroecología. In: Llorente–Bousquets J. and Morrone J.J. Eds. Introducción a la Biogeografía en Latinoamérica: Teorías, Conceptos, Métodos y Aplicaciones, pp. 63–80, Las Prensas de Ciencias, Universidad Nacional Autónoma de México, Mexico City. [ Links ]
Balvanera P, Lott E., Segura G., Siebe C. and Islas A. 2002. Patterns of B–diversity in a Mexican tropical dry forest. Journal of Vegetation Science 13:145–158. [ Links ]
Barthlott W. and Porembski S. 2000. Vascular plants on inselbergs: systematic overview. In: Porembski S. and Barthlott W. Eds. Inselbergs: Biotic Diversity of Isolated Rock Outcrops in Tropical and Temperate Regions, pp. 103–116, Springer–Verlag, Berlin. [ Links ]
Becerra J.X. 2005. Timing the origin and expansion of the Mexican tropical dry forest. Proceedings of the National Academy of Sciences USA 102:10919–10923. [ Links ]
Borhidi A. 1996. Phytogeography and Vegetation Ecology of Cuba. Akadémiai Kiadó, Budapest. [ Links ]
Burke A. 2003. Inselbergs in a changing world – global trends. Diversity and Distributions 9:375–383. [ Links ]
Cevallos–Ferriz S.R. and Ramírez J.L. 2004. Bosquejo de la evolución florística. In: García Mendoza A.J., Ordóñez M.J. and Briones–Salas M. Eds. Biodiversidad de Oaxaca, pp. 87–104, Instituto de Biología, Universidad Nacional Autónoma de México, Fondo Oaxaqueño para la Conservación de la Naturaleza and World Wildlife Fund, Mexico City [ Links ]
Chain–Guadarrama A. 2005. Síndromes de dispersión en el mosaico vegetacional de la región de Nizanda (Oaxaca), México. B.Sc. Thesis, Facultad de Ciencias, Universidad Nacional Autónoma de México, Mexico City, 67 pp. [ Links ]
Cox B. 2001. The biogeographic regions reconsidered. Journal of Biogeography 28:511–523. [ Links ]
Durán E., Meave J.A., Lott E.J. and Segura G. 2006. Structure and diversity patterns at landscape level in a Mexican tropical deciduous forest. Boletín de la Sociedad Botánica de México 79:43–60. [ Links ]
Eiten G. 1986. Use of term "savanna". Tropical Ecology 27:10–22. [ Links ]
Felfili M.C. and Felfili J.M. 2001. Diversidade alpha e beta no cerrado sensu stricto da Chapada Pratinha, Brasil. Acta Botânica Brasílica 15:243–254. [ Links ]
Furley P 2006. Tropical savannas. Progress in Physical Geography 30:105–121. [ Links ]
Gallardo–Cruz J.A., Meave J.A. and Pérez–García E.A. 2005. Composición y diversidad de la selva baja caducifolia del Cerro Verde, Nizanda (Oaxaca), México. Boletín de la Sociedad Botánica de México 76:19–35. [ Links ]
Gallardo–Cruz J.A., Pérez–García E.A. and Meave J.A. 2009. B–diversity and vegetation structure as influenced by slope aspect and altitude in a seasonally dry tropical landscape. Landscape Ecology. 24: 473–482. [ Links ]
Gentry A.H. 1995. Diversity and floristic composition of Neotropical dry forests. In: Bullock S.H., Mooney H.A. and Medina E. Eds. Seasonally Dry Tropical Forests, pp. 146–194, Cambridge University Press, Cambridge. [ Links ]
Halffter G., Soberón J., Koleff P. and Melic A. (Eds.) 2005. Sobre Diversidad Biológica: el Significado de las Diversidades Alfa, Beta y Gamma. Sociedad Entomológica Aragonesa. Zaragoza, Spain. [ Links ]
Hartshorn G.S. 2002. Biogeografía de los bosques neotropicales. In: Guariguata M.R. and Kattan G.H. Eds. Ecología y Conservación de Bosques Neotropicales, pp. 59–81, Libro Universitario Regional, Cartago, Costa Rica. [ Links ]
Ivanausckas N.M. and Rodrigues R.R. 2000. Florística e fitosociologia de remanescentes de floresta estacional decidual em Piracicaba, São Paulo, Brasil. Revista Brasileira de Botânica 23:291–304. [ Links ]
Kent M. and Coker P. 2003. Vegetation Description and Analysis: A Practical Approach. John Wiley & Sons, New York. [ Links ]
López–Olvera I. 2005. Patrones de morfología foliar y formas de crecimiento de las Fabaceae en la región de Nizanda (Oaxaca), México. B.Sc. Thesis, Facultad de Ciencias, Universidad Nacional Autónoma de México, Mexico City, 64 pp. [ Links ]
Lott E.J. and Atkinson T.H. 2006. Mexican and Central American seasonally dry forests: Chamela–Cuixmala, Jalisco, as a focal point for comparison. In: Pennington T.T., Lewis G.P and Ratter J.A. Eds. Neotropical Savannas and Seasonally Dry Forests: Plant Diversity, Biogeography, and Conservation, pp. 315–342, The Systematic Association, Boca Raton, Fl. [ Links ]
Magurran A.E. 2004. Measuring Biological Diversity. Blackwell Publishing. Oxford. [ Links ]
Martínez–Yrízar A., Búrquez A. and Maass M. 2000. Structure and functioning of tropical deciduous forest in Western Mexico. In: Robichaux R.H. and Yetman D.A. Eds. The Tropical Deciduous Forests of Los Alamos: Biodiversity of a Threatened Ecosystem in Mexico, pp. 19–35. The University of Arizona Press, Tucson. [ Links ]
Menaut J.C., Lepage M. and Abbadie L. 2005. Savannas, woodlands and dry forests in Africa. In: Bullock S.H., Mooney H.A. and Medina E. Eds. Seasonally Dry Tropical Forests, pp. 64–92, Cambridge University Press, Cambridge. [ Links ]
Miles L., Newton A.C., DeFries R.S., Ravilious C., May I., Blyth S., Kapos V. and Gordon J.E. 2006. A global overview of the conservation status of tropical dry forests. Journal of Biogeogra–phy 33:491–505. [ Links ]
Miranda F. and Hernández–X. E. 1963. Los tipos de vegetación en México y su clasificación. Boletín de la Sociedad Botánica de México 28:29–179. [ Links ]
Mooney H.A., Bullock S. and Medina E. 1995. Introduction. In: Bullock S.H., Mooney H.A. and Medina E. Eds. Seasonally Dry Tropical Forests, pp. 1–8, Cambridge University Press, Cambridge. [ Links ]
Murphy P.G. and Lugo A.E. 1986. Ecology of tropical dry forest. Annual Review of Ecology and Systematics 17:67–88. [ Links ]
Murphy P.G. and Lugo A.E. 1995. Dry forests of Central America and the Caribbean. In: Bullock S.H., Mooney H.A. and Medina E. Eds. Seasonally Dry Tropical Forests, pp. 9–34, Cambridge University Press, Cambridge. [ Links ]
Oliveira P.S. and Marquis R.J. (Eds.) 2002. The Cerrados of Brazil: Ecology and Natural History of a Neotropical Savanna. Columbia University Press, New York. [ Links ]
Pavleti Z. and Trinajstic I. 1997. Rocky plant vegetation of the ass. Campanulo–Centaureetum dalmaticae H–ic (1934) 1939 (Centaureo–Campanulion H–ic) (1934) on the island of Cres (Croatia). Periodicum Biologorum 99:441–443. [ Links ]
Z. and Trinajstic I. 1997. Rocky plant vegetation of the ass. Campanulo–Centaureetum dalmaticae H–ic (1934) 1939 (Centaureo–Campanulion H–ic) (1934) on the island of Cres (Croatia). Periodicum Biologorum 99:441–443. [ Links ]
Pennington R.T., Prado D.E. and Pendry C.A. 2000. Neotropical seasonally dry forest and Quaternary vegetation changes. Journal of Biogeography 27:261–273.
Pennington R.T., Lavin M., Prado D.E., Pendry C.A., Pell S.K. and Butterworth C.A. 2004. Historical climate change and speciation: neotropical seasonally dry forest plants show patterns of both Tertiary and Quaternary diversification. Philosophical Transactions of the Royal Society of London, series B 359:515537. [ Links ]
Pérez–García E.A. and Meave J.A. 2004. Heterogeneity of xerophytic vegetation of limestone outcrops in a tropical deciduous forest region. Plant Ecology 175:147–163. [ Links ]
Pérez–García E.A. and Meave J.A. 2006. Coexistence and divergence of tropical dry forests and savannas in southern Mexico. Journal of Biogeography 33:438–447. [ Links ]
Pérez–García E.A., Meave J. and Gallardo C. 2001. Vegetación y flora de la región de Nizanda, Istmo de Tehuantepec, Oaxaca. Acta Botanica Mexicana 56:19–88. [ Links ]
Pérez–García E.A., Meave J.A. and Gallardo–Cruz J.A. 2005. Diversidad β y diferenciación florística en un paisaje complejo del trópico estacionalmente seco del sur de México. In: Halffter G., Soberón J., Koleff P. and Melic A. Eds. Sobre Diversidad Biológica: el Significado de las Diversidades Alfa, Beta y Gamma, pp. 123–142, Sociedad Entomológica Aragonesa, Zaragoza, Spain. [ Links ]
Porembski S., Brown G. and Barthlott W. 1995. An inverted latitudinal gradient of plant diversity in shallow depressions on ivo–rian inselbergs. Vegetatio 117:151–163. [ Links ]
Porembski S., Martinelli G., Ohlemüller R. and Barthlott W. 1998. Diversity and ecology of saxicolous vegetation mats on insel–bergs in the Brazilian Atlantic rainforest. Diversity and Distributions 4:107–119. [ Links ]
Prance G.T. 2006. Tropical savannas and seasonally dry forests: an introduction. Journal of Biogeography 33:385–386. [ Links ]
Romero–Centeno R. and Zavala–Hidalgo J. 2003. Isthmus of Tehu–antepec wind climatology and ENSO signal. Journal of Climate 16:2628–2638. [ Links ]
Rzedowski J. 1962. Contribuciones a la fitogeografía florística e histórica de México I. Algunas consideraciones acerca del elemento endémico en la flora mexicana. Boletín de la Sociedad Botánica de México 27:52–65. [ Links ]
Rzedowski J. 1991. Diversidad y orígenes de la flora fanerogámica de México. Acta Botanica Mexicana 14:3–21. [ Links ]
Sampaio E.V. S.B. 1995. Overview of the Brazilian caatinga. In: Bullock S.H., Mooney H.A. and Medina E. Eds. Seasonally Dry Tropical Forests, pp. 35–63, Cambridge University Press, Cambridge. [ Links ]
Salas–Morales S.H., Saynes–Vásquez A. and Schibli L. 2003. Flora de la costa de Oaxaca, México: lista florística de la región de Zimatán. Boletín de la Sociedad Botánica de México 72:21–58. [ Links ]
Scariot A. and Sevilha A.C. 2005. Biodiversidade, estrutura e conservação de florestas estacionais deciduais no cerrado. In: Scariot A., Sousa–Silva J.C. and Felfili J.M. Eds. Ecologia, Biodiversidade e Conservação do Cerrado, pp. 121–139, Ministério do Meio Ambiente, Brasilia. [ Links ]
Seine S., Porembski S. and Becker U. 2000. Phytogeography. In: Porembski S. and Barthlott W. Eds., Inselbergs: biotic diversity of isolated rock outcrops in tropical and temperate regions. pp. 435–449, Springer–Verlag, Berlin. [ Links ]
Silva L.Á. and Scariot A. 2003. Composição florística e estrutura da comunidade arbórea em uma floresta estacional decidual em afloramento calcário (Fazenda São José, São Domingos, GO, Bacia do Rio Paranã). Acta Botânica Brasílica 17:305–313. [ Links ]
Silva L.Á. and Scariot A. 2004a. Comunidade arbórea de uma floresta estacional decídua sobre afloramento calcário na Bacia do Rio Paraná. Revista Arvore 28:61–67. [ Links ]
Silva L.Á. and Scariot A. 2004b. Composição e estrutura da comunidade arbórea de uma floresta estacional decidual sobre afloramento calcário no Brasil central. Revista Arvore 28:69–75. [ Links ]
Silva, M.F.F., Secco, R.S. and M.G.A. Lobo. 1996. Ecological aspects of the tropical scrub vegetation on rocky outcrops of the Serra dos Carajas, state of Para, Brazil. Acta Amazónica 26:1744. [ Links ]
StatSoft Inc. 1995. STATISTICA for Windows. Tulsa. [ Links ]
Torres–Colín L.M. 1989. Estudio florístico y descripción de la vegetación del Cerro Guiengola, en el Istmo de Tehuantepec, Oaxaca. B.Sc. Thesis, Escuela Nacional de Estudios Profesionales Iztacala, Universidad Nacional Autónoma de México. Los Reyes Iztacala, Mexico, 81 pp. [ Links ]
Trejo I. and Dirzo R. 2002. Floristic diversity of Mexican seasonally dry tropical forests. Biodiversity and Conservation 11:2063–2084. [ Links ]
Vane–Wright R.I., Humphries C.J. and Williams P.H. 1991. What to protect? Systematics and the agony of choice. Biological Conservation 55:235–254. [ Links ]
Walter H. 1973. Die Vegetation der Erde: in Oko–physiologischer Betrachtung. Band I: Die Tropischen und Subtropischen Zonen. Gustav Fischer Verlag, Stuttgart. [ Links ]
Wendt T. 1998. Composición, afinidades florísticas y orígenes de la flora arbórea del dosel de los bosques tropicales húmedos de la vertiente mexicana del Atlántico. In: Ramamoorthy T.P, Bye R., Lot A. and Fa J. Eds. Diversidad Biológica de México. Orígenes y Distribución, pp. 581–664, Universidad Nacional Autónoma de México, Mexico City. [ Links ]
Wilson M.V. and Shmida A. 1984. Measuring beta diversity with presence–absence data. Journal of Ecology 72:1055–1064. [ Links ]
Whittaker R.H. 1960. Vegetation of the Siskiyou Mountains, Oregon and California. Ecological Monographs 30:279–338. [ Links ]














