Introduction
Production of a monosex population in tilapia culture through hormone sex reversal eliminates uncontrolled reproduction and allows the production of marketable-sized fish (Varadaraj, 1989). All-male tilapia show better growth compared with females, and for many years the production of all-male tilapia has been recognized as the most effective technique to increase tilapia production (Vera-Cruz et al., 1996; Mair et al., 1997; Müller & Hörstgen, 2007; Nonglak et al., 2012). Although with Nile tilapia it is feasible to obtain close to 100% males by feeding the fry with different hormones, failure to optimize the necessary parameters such as age and size of the fry and feeding frequency can commonly result in lower than expected rates of sex reversal (Mair et al., 1995; Abucay & Mair, 1997). Additionally, this method is increasingly being contested since there is a growing concern about hormones in the environment, and an increasing number of consumers are interested in environmentally friendly production techniques and do not want to eat products that have been treated with hormones or other active substances (Piferrer, 2001; Müller & Hörstgen, 2007; Leet et al., 2011).
Application in recent years of a newer genetic technique that allows for the production of all-male progeny by crossing the so-called supermales that possess a novel homogametic "YY" genotype with normal females (XX) can be considered a viable alternative on a commercial scale (Vera-Cruz et al., 1996; Mair et al., 1997; Müller & Hörstgen, 2007). The initial development of YY males requires feminization of XY genotypes during their sexually undifferentiated stage and the identification of these newly created "sex-reversed females" (females XY) through a progeny test (Mair et al., 1997). Although there have been numerous published attempts to optimize feminization of genotypically XY O. niloticus (Linnaeus, 1758) by varying parameters such as hormone dose, treatment start time, duration of treatment and stocking density, this method still has some limitations (Mair & Santiago, 1994; Rosenstein & Hulata, 1994; Vera-Cruz et al., 1996; Piferrer, 2001). Additionally, there is little information about the effects of estradiol-17β (E2) on growth after treatment, gonadal development and body composition in sex-reversed individuals.
The present study was undertaken to (1) determine whether high proportions of sexually undifferentiated XY O. niloticus fry could be sex-reversed to functional females using E2 and (2) describe the effect of E2 on the feminization of the gonads, on growth and on body composition after normal sex differentiation in cultivated tilapia. This is a preliminary step towards our ultimate objective of developing a breeding program to produce a male genetic population through supermale (YY) in the southern region of Mexico, one of the highest producers of tilapia.
Material and methods
Fish. The O. niloticus broodstock used in this research originates from the Centro Acuícola de Temazcal (Oaxaca, Mexico) and the Sistema Cooperativo Integral (Veracruz, Mexico). This broodstock has been acclimated for a year in the experimental aquaculture station of the Universidad del Papaloapan and fed with commercial floating pellets (25% protein, Nutripec, Agribrands Purina, Irapuato Gto. Mexico).
Hormonal treatment. Synthetic hormone E2 (Sigma, Sigma-Aldrich Chemical Co., St Louis, MO, USA) was added to the commercial fish meal (protein 50%, lipids 15%, fiber 2.5%, ash 12%, moisture 12%, N,-free extract 8.5%) using the alcohol evaporation method of Guerrero (1975). Briefly, E2 was dissolved in 95% ethanol, sprayed over the food and kept overnight at room temperature to evaporate the alcohol. Two E2 hormone treatment feeds were prepared, 60 mg kg-1 and 120 mg kg-1. Doses were selected according to their relative oral potency. The food for the control group was treated in exactly the same manner with the exclusion of the hormone.
Fry production and experimental conditions. O. niloticus broodstock were stocked at a male:female ratio of 1:3 in three 3-m diameter outdoor concrete tanks (28-30 ºC) supplied with fertilized water. Recently-hatched and sexually undifferentiated fry (~8 mm length) were collected 14 days later with a fine-mesh net after siphoning 90% of the water in the tanks. The fry were pooled, transported to a closed recirculating system and randomly divided into nine 85-L acrylic aquaria (three aquaria per treatment) at an initial stocking density of 4 fry/L. The water in the recirculating system was filtered with a mechanical filter (Hayward, Model S310T2, Hayward Pool Products Inc., Elizabeth, NJ, USA), with a bio-filter containing only plastic bio-balls (Aquatic Eco-System, Model CBB1, Pentair Ltd., Apopka, FL, USA) and UV-sterilized (Lumiaction, Model BE1X20, Lumiaction Co. Ltd, Taipei, Taiwan). During the period of treatment, a photoperiod of 12L: 12D was used and water temperatures were maintained at 28 ± 1 ºC.
Fry were fed ad libitum eleven times a day at 1-h intervals for 30 days. Water flow was closed in all aquaria for 20 min after the steroid-enriched feeds were offered in order to encourage feeding. Random samples of 30 fry per treatment replicate were taken every 10 d. Standard length was recorded to the nearest (0.01 mm) from a digitized image (ImageJ version 1.36. Water temperature and dissolved oxygen were monitored daily (YSI, model 655, Yellow Springs Instrument Co., Inc., Yellow Springs, OH, USA) (± 0.1), while pH (Thermo Scientific, Model Orion 3 Star, Thermo Fisher Scientific, MA, USA) and ammonium (Nutrafin, Rolf C Hagen Corp., MA, USA) were evaluated once a week. Aquaria were syphoned daily.
At the end of the experiment, all fry from each treatment replicate were counted and measured for the calculation of survival rate and average length. The fry were nursed in outdoor 1.2-m diameter floating net cages and fed ad libitum six times a day with untreated commercial diet (40% protein) for another 30 days and then transferred to 3-m diameter concrete tanks supplied with fertilized water and reared up to sexual maturity (five months of age). They were fed with untreated commercial diet (35% protein) three times of day and subsequently a commercial diet with 25% protein until the end of the experiment. The water temperature ranged from 28 to 31 ºC during all the post-treatment part of the experiment. Standard length and wet weight were registered every 21 d in 30 random individuals per treatment replicate.
Evaluation of sex ratio and identification of sex-reversed females. The sex of the fish in each treatment was determined by external examination using dye (methylene blue at 1%) to highlight the differences of the papilla structure. Each fish was classified as male, female, or atypical. In the atypical fish the papillae did not have a normal appearance. Approximately 50 fish per sex (including atypical) were weighed, measured and sexed again by removing the gonad and the spermatic/ovarian ducts. Before extraction, all fish received a gentle abdominal massage in order to determine the precise site of expulsion of the gametes (oviduct or male vas deferens). Gonads were classified as ovaries, testes or intersex. Individuals having female papilla and ovaries were then classified as typical females, while individuals having male papilla and testes were classified as typical males. Fish that had atypical papilla and ovaries were then classified as sex-reversed females (genetic males).
Histology. The gonads were preserved for 48-h in a Davidson solution before they were fixed in a 4% formalin solution. They were embedded in paraffin, cut into 5 µm sections and stained with hematoxilin-eosin solution for light microscopic observation.
Body Composition. Analyzes were made on muscle samples obtained from the lateral part of the fish. The muscle samples were cut in slices (5-10 g), oven dried at 120 ºC for 24 h and homogenized with the aid of an industrial blender, followed by fat and protein analysis. Protein was determined by the method 954.01 (AOAC, 1997) and fat contents were measured using the method 920.39 (AOAC, 1997).
Statistics. Differences in standard length and wet weight were analyzed using a one-way analysis of variance. The sex ratio (% female), percentage of survival and differences in body composition between the different treatments were arcsine-transformed prior to analysis using a one-way analysis of variance (Zar, 1974).
Results
Fry. Survival and standard length of treated and control fry during E2 treatment is shown in Table 1. No differences were detected in survival between the E2 treated groups and the control group. Standard length was significantly (p < 0.05) lower in the dose of 60 mg kg-1 in the last two weeks of the E2 treatment compared with the control group and the dose of 120 mg kg-1. There were no significant differences between the control group and the dose of 120 mg kg-1.
Table 1 Mean survival and standard length* (± S.E.) (n = 3) of Nile tilapia treated with estradiol-17β at different doses.
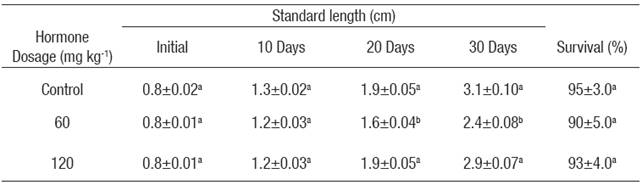
*Values in each column superscripted with different letters indicate significant differences (p < 0.05).
Sex ratio. Sex-reversed females were produced in both E2 treatments. The proportion of males was significantly lower (p < 0.05) in both E2 treatments compared to the control group (Table 2). Hormone concentration had a significant effect on the production of sex-reversed females, with a significantly (p < 0.05) higher production in the dose of 120 mg kg-1 compared with the dose of 60 mg kg-1 (Table 2). Only one intersex individual was detected in the dose of 60 mg kg-1 by the presence of testicular tissue between oocytes in various stages of development.
Table 2 Mean survival (± S.E.) (n = 3) and sex distribution* of Nile tilapia treated with estradiol-17β at different doses for 30 days. Data collected at the end of experiment (five months of age).
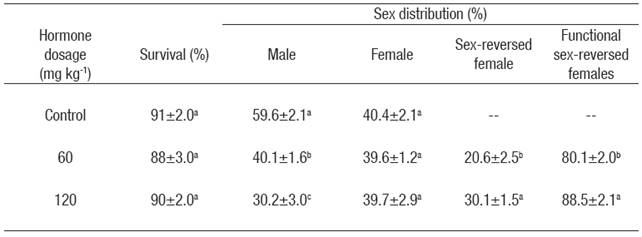
*Values in each column superscripted with different letters indicate significant differences (p < 0.05).
In this study, atypical fish had a papilla very similar to the papilla of males, but shorter. No obvious oviduct was observed. Extraction of the gonad revealed that fish with atypical papilla had ovaries. Atypical fish were therefore considered sex-reversed females and not counted as females. But only the atypical fish in which it was possible to obtain a sample of eggs in perfect condition after an abdominal massage were considered functional sex-reversed females (Table 2). Further analysis revealed that eggs obtained from atypical fish were expelled through a quasi-oviduct near the male vas deferens or through the male vas deferens. Fish classified as male or female had normal looking testicles or ovaries, respectively.
Survival and growth. No differences were detected post-treatment on survival up to sexual maturity between the E2 treated and the control groups (Table 2). There were significant (p < 0.05) differences in the mean wet weight and standard length between sexes in all groups analyzed, with males being significantly heavier (Figure 1a) and larger (Figure 1b) in the control and in the E2 treated groups. No differences were observed between treated females and sex-reversed ones in any of the E2 treated groups (Figure 1a, b). The mean wet weight of males in the dose of 60 mg kg-1 was significantly (p < 0.05) heavier compared with the males of the control group. Females of the dose of 120 mg kg-1 showed a significantly (p < 0.05) lower mean wet weight than the females of the control group. There were no significant differences in the standard length between the E2 treated and control groups.
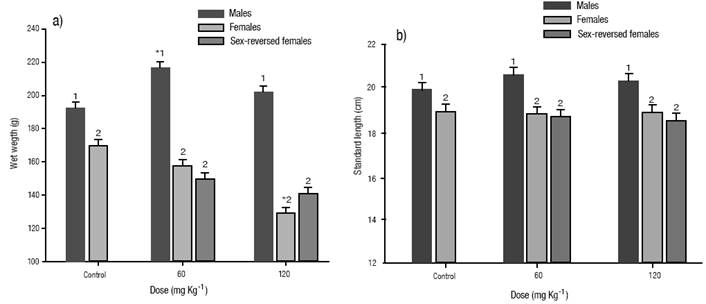
Figures 1a-b a) Growth at five months of age of Nile tilapia treated with estradiol-17β at different doses for 30 days. (a) wet weight. b). Standard length. The vertical bars represent the mean ± S.E., of three replicates. Significantly different from the control group: *p < 0.05. Values in each column superscripted with different numbers indicate significant differences between sexes (p < 0.05).
Histology. Sections of testis of the control group showed normal testicular tissue architecture and sperm cell distribution (Fig. 2a). Males fed with E2 showed no differences in tissue architecture or cell development compared with the control group (Fig. 2b). However, in general, a lower number of spermatozoids were observed inside the seminiferous tubules. Histological sections of the control and E2 treated females showed normal ovary histology characterized by the presence of oocytes in advanced vitellogenesis (Figs. 2c-d), while sex-reversed females showed a gonadal development characterized by the presence of oocytes in initial, mid and advanced vitellogenesis (Figs. 2e-f). Further observation did not reveal testicular tissue in any of the sex-reversed females analyzed.

Figures 2a-f Transverse section of gonads. (a) Testis of a male of the control group and (b) testis of an E2 treated male, both showing normal seminiferous tubule (arrow) and cell development, (c) ovary of a female of the control group and (d) ovary of an E2 treated female both characterized by the presence of oocytes in advan ced vitellogenesis (arrow), (e) ovary of a sex-reversed female characterized by oocytes in initial and mid vitellogenesis (arrow) and (f) ovary of a sex-reversed female characterized by oocytes in advanced vitellogenesis (arrow). Panels a and b are x10 and c-f are x20.
Body Composition. Fat in the muscle increased significantly (p < 0.05) in the sex-reversed females of both E2 treated groups compared to the control group (Fig. 3a). E2 treated females of the dose of 120 mg kg-1 showed the lowest value of fat in muscle (p < 0.05). No differences were detected between treated males and females of the dose of 60 mg kg-1 and the control group. In contrast, protein in the muscle was reduced significantly (p < 0.05) in the sex-reversed females of both E2 treated groups compared to the control group (Fig. 3b). E2 treated fish of the dose of 120 mg kg-1 showed a similar reduction in the content of protein compared to the fish in the control group (p < 0.05). The highest percentage of protein in muscle was detected in the treated females of the dose 60 mg kg-1 (p < 0.05). In the control group, the females showed a significantly higher (p < 0.05) protein percentage than males. In the E2 treated groups, females of the dose of 60 mg kg-1 showed a significantly higher (p < 0.05) percentage than males and sex-reversed females, while males showed a significantly higher (p < 0.05) percentage than females and sex-reversed females in the dose of 120 mg kg-1 (Fig. 3b).
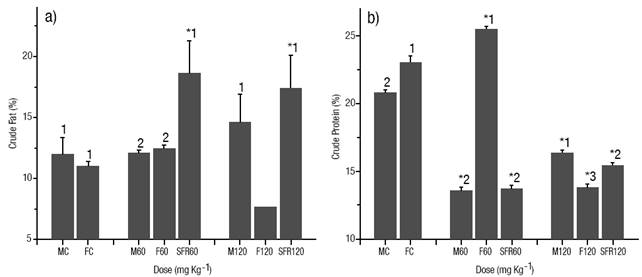
Figures 3a-b Body composition of the muscle (% of dry weight). (a) Crude fat. (b). crude protein. The vertical bars represent the mean ± S.E. of three replicates. MC male of the control group, FC female of the control group, M60 male treated at a 60 mg kg-1 dose, F60 female treated at a 60 mg kg-1 dose, SRF60 sex-reversed female of the dose of 60 mg kg-1, M120 hormone treated male at a 120 mg kg-1 dose, F120 hormone treated female at a 60 mg kg-1 dose, SRF120 sex-reversed female of the dose of 120 mg kg-1. Significantly different from the control group: *p < 0.05. Values in each column superscripted with different numbers indicate significant differences between sexes (p < 0.05).
Discussion
The effect of E2 concentration on production of sex-reversed females was clearly dose-dependent in our experiment. Increase of sex-reversed females was superior compared to that reported in previous works for other tilapia species using E2 (Hopkins et al., 1979; Jensen & Shelton, 1979; Bombardelli & Hayashi, 2005). The better efficiency of sex-reversal observed in our study could be related to the fact that the fry used had an average length of 8 mm at the first feeding. Vera-Cruz et al. (1996) recommend for Nile tilapia that the fry used should have an initial length of less than 10 mm at the first feeding to ensure a successful feminization. According to Melard (1995) suitable rearing conditions and continuous feeding over a 12-h period may improve the appetite and allow for a better assimilation of E2. Additionally, Abucay and Mair (1997) suggest that sex reversal treatments will probably be more successful in recirculating water systems where metabolites or leachates can build up rather than in flow-through systems where these would be lost.
Despite the apparent improved assimilation of E2, hormone treatment neither affected survival nor altered growth rate of fry. It has been reported that estrogen has no effect on growth in fish, unlike androgen (Johnstone et al., 1979; Piferrer, 2001). However, several authors have observed positive growth using synthetic and natural estrogen (Cowey et al., 1973; Yu et al., 1979; Varadaraj, 1989; Degani, 1986; Chiba et al., 1993). A negative effect has also been observed, but only in higher doses (Varadaraj, 1989; Melard, 1995; Piferrer, 2001). The lower growth observed at the end of the E2 treatment in the dose of 60 mg kg-1 was probably a result of a formalin treatment applied for controlling a possible outbreak of trichodiniasis (Trichodina sp.). This prophylactic treatment was applied to all groups two weeks after the start of the experiment, but only the fry of the dose of 60 mg kg-1 showed a temporary reduction in food intake after the treatment.
The differences in growth observed post-treatment between males and females (including sex-reversed females) were probably associated with the sexual dimorphism of this species rather than an effect of the E2 treatment. Toguyeni et al. (1996) report that males of Nile tilapia show higher growth rates than females, which may be directly related to sex hormones or indirectly through sex-related behavior (mouthbrooding) and physiological factors (a higher metabolic energy output required for egg production) (Nonglak et al., 2012). In our work, no significant differences were found in growth between sex-reversed fish and female fish. Similar growth between sex-reversed fish and normal ones has been previously reported in the Nile tilapia (Nonglak et al., 2012) and the Mozambique tilapia Oreochromis mossambicus Peter, 1852 (Sparks et al., 2003). Magurran and García (2000) report that growth is a sex-related characteristic regulated by sex hormones and is influenced by both genetics and the environment and thereby displayed after sex differentiation. Therefore, a hormone treatment applied before sex differentiation would modify growth patterns in the sex-reversed fish. Similar growth patterns between normal females and sex-reversed ones would indicate a complete feminization at metabolic and hormonal levels.
Several authors have reported the presence of atypical fish after a feminization/masculinization process (Hopkins et al., 1979; Jensen & Shelton, 1979; Calhoun & Shelton, 1983; Bye & Lincoln, 1986; Feist et al., 1995; Potts & Phelps, 1995). Atypical fish are characterized by having abnormal papillae or sometimes for having ovaries but a male papilla. In general, these fish are discarded because they are considered not to possess a functional oviduct. In this work, atypical fish were considered sex-reversed females, but only fish from which we obtained a viable sample of eggs via abdominal massage were considered functional females and selected as breeders for the next step in the production of YY males (Mair et al., 1997). At this point, nine spawns have been obtained crossing atypical fish with normal males, of which approximately 90% have yielded a percentage of males close to 75%, indicating the presence of YY males (Alcántar-Vázquez et al., 2014). It is possible that the feminization process was complete at a physiological level in the atypical fish, but the channels associated with the oviduct were not properly formed and the papillae retained their male form. Gimeno et al. (1998) report in the common carp, Cyprinus carpio Linnaeus, 1758, that 20 days of exposure to E2 were sufficient to induce the formation of an oviduct instead of a male vas deferens. Although the duration of our treatment was longer and achieved a complete physiological change, low levels of hormone uptake or the interaction with environmental variables (water temperature) could result in an incomplete feminization at morphological level. This seems supported by the fact that some atypical fish had well-developed ovaries (identified after extraction), but abdominal massage failed to obtain an egg sample. In some cases, it was possible to observe atypical fish with a distended abdomen. Extraction of the gonad revealed an oversized ovary, a large accumulation of extracellular liquid and in some cases broken ovarian tissue with eggs released into the visceral cavity. Further analysis revealed that in these fish the male vas deferens was absent and the oviduct was not properly formed, as a consequence of an incomplete transition from male to female papilla. This situation probably leaves the oocytes without a proper channel to be expelled. Feist et al. (1995) report similar results in the rainbow trout Oncorhynchus mykiss Walbaum, 1792, in which a case high proportion sex-reversed males were produced through both immersion and feeding of 17β-methyltestosterone. However, the majority of the animals lacked or had incomplete sperm ducts and semen had to be removed surgically. In our work, in the cases where a sample of eggs was obtained through abdominal massage, this process resulted in crushed eggs in approximately 10% of the cases. This probably was the result of the eggs being expelled through a quasi-oviduct not properly formed or through a remaining male vas deferens not suitable for expelling eggs.
Wang and Tsai (2000) report that exposure of 10 day-old fry to elevated water temperatures (28-32 °C) induces gonadal masculinization and therefore an increase in the proportion of males. In our work, water temperature ranged from 27 to 29 ºC (28 ± 1 ºC) during all the E2 treatment, so it is probable that temperature can account for the elevated proportion of males observed in the control group. Similar results have been observed by Contreras-Sánchez (2001) in control groups of Nile tilapia reared at a water temperature of 28 ± 1 ºC during a masculinization process. These results suggest that a genetic tendency toward the masculinization of the gonads at moderately elevated temperatures could be responsible for the proportion of males observed. Wang and Tsai (2000) suggest that sex differentiation is a temperature-dependent mechanism, and that male-producing temperatures would be up-regulating the production of reductase and/or androgen receptor and in turn switching to male development. Baroiller et al. (2009)) mention that this is related to the interactions of the three components that govern sex in the Nile tilapia, a complex genetic sex determination system with a major determinant locus (sex chromosomes XX/XY) and some minor genetic factors (parental factors), as well as the influence of temperature (environmental factors).
Considering the sensivity to temperature observed in the control group, it is probable that the treated groups were also affected by the water temperature maintained during the E2 treatment. Although synthetic hormones 17-α-ethynylestradiol and diethylstilbestrol have shown higher feminization rates than the natural hormone E2 in similar species, we expected a higher proportion of sex-reversed females feeding the fry ad libitum at 1-h intervals for 30 days. Our results suggest that this sensitivity to water temperature could be as important during a feminization process as the feeding regime or the duration of the hormone treatment. Early experiments carried out in our laboratory have already shown a significant increase in the female proportion combining the water temperature with an appropriate dose of E2 (unpublished results). Further research in this aspect will be the next step to increase the number of females obtained during a feminization process.
Gonadal development was normal in all fish analyzed, indicating that the dosage used in the present study was physiologically adequate. Several authors report negative effects on male gonads after a sustained exposure to E2 or similar substances (Billard et al., 1981; Gimeno et al., 1998; Panter et al., 1998). In our case, only a slight reduction in the number of spermatozoa was observed in the hormone-treated males. It appears that the negative effects of E2 on male development are highly dependent on the species and hormone concentration. Hopkins et al. (1979) report in the Blue tilapia, Tilapia aurea Steindachner, 1864, the presence of abnormal gonads after a prolonged treatment with different estrogens in 94% of atypical fish. In this work, sex-reversed females showed a normal gonadal development with oocytes in various stages of development, but no testicular tissue. This indicates that hormone concentration and duration of treatment were adequate to successfully complete feminization at a physiological level, although the channels associated with oviduct were not properly formed in a high percentage of the sex-reversed females observed.
Degani (1986) and Chiba et al. (1993) report an increase in the amount of fat in muscle and a reduction of protein content following E2 treatment in the European eel, Anguilla anguilla Linnaeus, 1758 and the Japanese eel, Anguilla japonica Temmnick & Schlegel, 1846 respectively. In our study, increase of fat content was particularly marked in the sex-reversed females, while reduction of protein in muscle was similar in all treated groups compared to untreated control, except for the females of the dose of 60 mg kg-1. Chiba et al. (1993) suggest that E2 may play a role in the regulation of protein and lipid metabolism in the muscle of fish. Olivereau and Olivereau (1979)) and Haux and Norberg (1985) report a depletion of liver glycogen and an increase in protein content after repeated intramuscular injections of E2 in females of freshwater eel and juveniles of rainbow trout respectively. It is probable that a sustained treatment (oral or intramuscular) of E2 provokes opposite metabolic effects on liver and muscle. Increase of fat in muscle is probably caused by the accumulation of glycogen and other lipid products of an excessive stimulation of E2 on the liver. Panter et al. (1998) report an increase in vitellogenin synthesis after exposure to E2 in the fathead minnow, Pimephales promelas Rafinesque, 1820. Finally, Hoar et al. (1983) and Nagahama (1994) suggest that during sexual maturation, E2 acts on the liver to stimulate the production of vitellogenin. This supports the idea that the increase of fat in muscle is probably related to the action of E2 on the liver. Reduction of protein content must be linked to a change in the metabolic pathways between muscle and liver. The increase in the ability of the liver to synthesize new proteins (Haux & Norberg, 1985) after an E2 treatment supports this fact.
The successful feminization of the XY genotype is a vital step in the development of the YY technology. Although in our study the proportion of sex-reversed females with functional oviduct was lower than expected by using a closed recirculating system and increasing the feeding rate, we still produce a sufficient number of functional sex-reversed females to make possible the production of YY males through the mating of sex-reversed females (XY) with normal males (XY). However, in succeeding studies, it will be necessary to combine the use of hormones with water temperatures that favor a higher proportion of females in order to optimize the number of sex-reversed females obtained and to achieve a complete feminization of the genital papilla. Additionally, comparative studies of the physiology and sexual behavior (currently under development) of sex-reversed females could help us understand the changes caused by the administration of this hormone in the subsequent stages of development of the fish, this with the objective of developing this technology and allowing for its establishment on a commercial scale in this region.











 nueva página del texto (beta)
nueva página del texto (beta)


