ANNEX 1
HEALTH DEPARTAMENT. GENERAL HOSPITAL MANUEL GEA GONZALEZ. INFORMED CONSENT LETTER
In accordance with the Helsinki Declaration principles, and with the General Health Law, on Ethical Aspects of Research in Human Beings, CHAPTER I, common provisions, articles 13 and 14. In all research where the human being is subject of study, the criterion of respect to dignity must prevail as well as protection of his rights and welfare. This research was considered of minimum risk in accordance with article 17 and in compliance with the following issues mentioned en Article 21
I. It has been explained to me that I suffer a jaw problem (temporomandibular dysfunction) and that it has been proposed to me to participate in a project where they will take measurements of my jaw.
II. I have been informed that models are going to be taken of my mouth with a material (alginate), and also, measurements of my bite will be taken with wa.
III. It has been explained to me that, when taking models with that material, if instructions are followed, there will be no problem. When a recording of my face is taken with a device which rests on my ears and my forehead, I can experience mild discomfort, for there will be pressure. This will be resolved when the device is withdrawn.
IV. Results of this study will help to determine a better treatment for temporomandibular dysfunction for myself as well as for other patients.
V. I have been given assurance that I can ask as much as I want about all things related to the study and my participation in it
VI. I have been informed that I can withdraw from the study whenever I decide, and this will not affect the service and attention of my treating physician or the hospital.
VII. I authorize publication or results of my study as long as professional discretion is upheld, my name will never be published or my identity revealed.
VIII. Physicians are committed to provide current information obtained during the stages of the study, even though this might affect the will of the participant to continue in the study
On _____________________ date, having understood what I am signing, and having all my doubts completely explained, I accept to participate in this study
<<Anterior-posterior and transverse variations in centric relation (CR) in patients with temporomandibular dysfunction (TD) and otologic symptoms (OS)>>

Two copies are drafted of this document, one is given to the subject of the research
(or his legal representative) and one is given to the researcher.

ANNEX 2
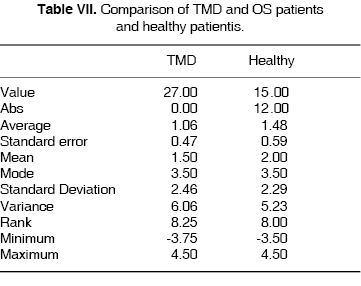
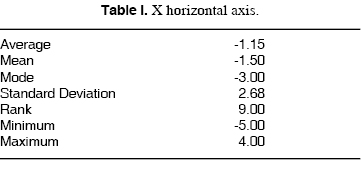
X HORIZONTAL AXIS


ANNEX 3
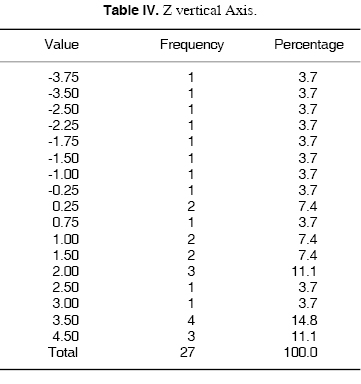
Z VERTICAL AXIS


ANEX 4
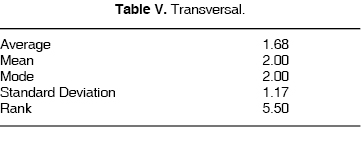
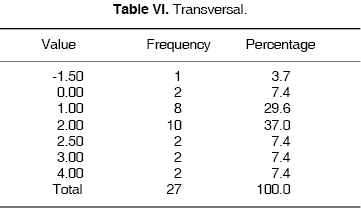
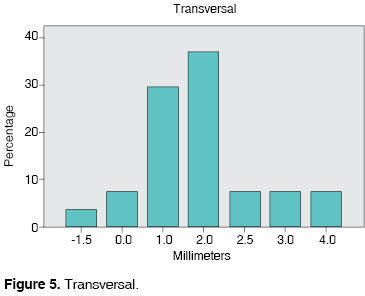
TRANSVERSAL